>Corresponding Author : Mohd Radzi Bin Hilmi
>Article Type : Research Article
>Volume : 2 | Issue : 1
>Received Date : 1 March, 2022
>Accepted Date : 14 March, 2022
>Published Date : 20 March, 2022
>DOI : https://doi.org/10.54289/JORVC2200105
>Citation : Rejab NSMD, Hilmi MRB, Kamal KM. (2022) Validation of IVCM In Measuring Sub-Basal Nerve Plexus and Keratocyte Cell Density in Corneal Wound Healing. J Ophthalmic Res Vis Care 2(1): doi https://doi.org/10.54289/JORVC2200105
>Copyright : © 2022 Rejab NSMD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access | Full Text
1School of Optometry, Faculty of Medicine and Health Sciences, Ucsi University, Cheras, Wilayah Persekutuan Kuala Lumpur
2Department of Optometry and Visual Science, Kulliyyah of Allied Health Sciences, International Islamic University Malaysia, Jln Sultan Ahmad Shah Bandar Indera Mahkota 25200 Kuantan, Pahang, Malaysia
3Department of Ophthalmology, Kulliyyah of Medicine, International Islamic University Malaysia, Jln Sultan Ahmad Shah Bandar Indera Mahkota 25200 Kuantan, Pahang, Malaysia
*Corresponding author: Mohd Radzi Bin Hilmi, Department of Optometry and Visual Science, Kulliyyah of Allied Health Sciences, International Islamic University Malaysia, Jln Sultan Ahmad Shah Bandar Indera Mahkota 25200 Kuantan, Pahang, Malaysia
Abstract
Introduction: This study aimed to evaluate the reliability measurement of corneal stromal thickness, sub-basal nerve plexus (SBNP) and keratocyte cell density (KCD) in laser refractive surgery patients.
Methods: 120 eyes of 60 participants were recruited and both right and left eyes of the myopic subjects were measured separately. Cornea stromal thickness were measured based on the cellular morphology that differs between each corneal layer. Measurement of SBNP and KCD were done using in-vivo confocal microscopy (IVCM) using corneal stromal thickness as reference. Corneal nerve parameters measured includes nerve fiber density (NFD), nerve branch Density (NBD) and nerve fiber length (NFL) while KCD were measured based the amount per area, depending on the region of interest. All images were captured and processed using ImageJTM Software and NeuronJ. All data were expressed in mean and standard deviation. Statistical analyses were performed using Predictive analytics software. P < 0.05 was set as the level of significance. Intra- and inter-observer intraclass correlation analysis were done to evaluate reliability of measurement in corneal stromal thickness, SBNP and KCD.
Results: This study found no significant difference between measurements for corneal stromal thickness, SBNP and KCD measured. (All P > 0.05). Intraclass correlation analysis showed both intra- and inter-observer performance were approximately consistent and reliable (All r > 0.90, P > 0.05).
Conclusion: Measurement of corneal stromal thickness, SBNP and KCD using IVCM is valid and reliable.
Keywords: In-vivo corneal confocal microscopy; Sub-basal nerve plexus; keratocyte cell density; myopia
Abbreviations: KCD: Keratocyte Cell Density, SBNP: Sub-Basal Nerve Plexus, IVCM: In-Vivo Confocal Microscopy, NFD: Nerve Fiber Density, NBD: Nerve Branch Density, NFL: Nerve Fiber Length, LRS: Laser Refractive Surgery, ROI, Region of Interest, QoL: Quality of Life IESC: Eye Specialist Clinic, IIUM: International Islamic University Malaysia, CN: Corneal Nerve, RCM: Rostock Cornea Module, CCD: Charge-Coupled Device, JPEG: Joint Photographic Experts Group, ICC: Intraclass Correlation Coefficient
Introduction
Currently, it is estimated that myopia prevalence would affect 4.5 billion people globally by 2050 [1, 2]. Rapid myopic transition is noted in Asians population were inevitable due to rapid economic transition with excessive near vision activities are moreprominent than outdoor activities, were found to be a critical factor [2]. Development of myopia can be due to elongation of the eyeball which known as axial myopia and could be due to changes in the structure or location of the image forming structures of the eye such as cornea and lens [3]. Recent study [4] had commented that young adult and children are more prone to develop myopia compared to elder group. Moreover, many studies had demonstrate the impact of myopia on quality of life (QoL) [5-8] in which clearly indicates higher prevalence of myopia would reduce QoL.
Laser refractive surgery (LRS) had been widely accepted as primary treatment of myopia apart from lens and contact lens. This is due to advancement in laser technology especially in late 20th century. It has been projected an increment of 4.7 million to 5.7 million surgical volume are projected from 2019 to 2024 [9], and it is expected to increase in future. Although there are significant advancement in LRS, it is worth to note that it does change the structural integrity and function of cornea. Sub-basal nerve plexus (SBNP) of the corneal nerve (CN) plays a pivotal role and and not restricted in detecting external stimuli, ocular surface protection, and maintaining ocular surface homeostasis [9,10]. On top of that, CN also plays role in corneal re-epithelialization by initiating or stimulating the production of several neuropeptides and corneal growth factors. Indirectly, CN is responsible for regulating the corneal epithelial proliferation, integrity, and wound healing process. Apart from SBNP, stromal ablation during LRS procedures could affect visual quality due to structural changes in keratocyte.
Keratocytes also play a significant role in corneal wound healing, structural and optical properties. Keratocytes consisted of fibroblast cells that are predominantly found in anterior corneal stroma. Reduction of Keratocytes cell density (KCD) was observed in many studies conducted on post-refractive surgery, contact lens wearing, corneal transplant and many more. Common symptoms reported by post-LRS patients includes dry eyes [11], neurotrophic epitheliopathy, or keratopathy [12] and loss of corneal sensitivity indicates involvement of transection of the corneal nerve plexus [9], and this has been postulated could be due to damage on the SBNP and KCD. To the best of our knowledge, limited evidence available that address the structural of both SBNP and KCD in pre-LASIK patients. This study aim to demonstrate reliability measurement of both SBNP and KCD using in-vivo corneal confocal microscopy approach in myopic eyes.
Materials and methods
120 eyes of 60 participants were recruited in this prospective cross-sectional study. Both right and left myopic eyes were measured separately. The inclusion criteria set for this study includes healthy participants age ranging from 19 to 25 years old for both male and female having spherical refractive error between -3.00D to -5.00D (moderate myopia) or more than -5.00D (high myopia) with maximum cylindrical error of -1.25DC, and maximum pupil size of 6.5 mm. All participants undergo laser refractive surgery, and within 3 months follow-up. The exclusion criteria are those with abnormal tear film [13-15] and corneal irregularity related conditions such as pterygium [15-23], history of ocular trauma and systemic diseases. Participants who worn soft contact lens within 2 weeks of the measurements, or 4 weeks for rigid gas-permeable contact lens were excluded [24-26].
Prior to commencement of study, ethical approval was obtained from IIUM Research Ethics Committee [IIUM/504/14/11/2/IREC 2019-KAHS (U)] and it is comform with the Tenets Declaration of Helsinki. All participants were given adequate information regarding methods and risks of this study. All participation are based on voluntary basis, and consent from each participants was obtained prior to any procedures. All data collection was conducted at IIUM Optometry Clinic, Kuliyyah of Allied Health Sciences, International Islamic University Malaysia (IIUM) Eye Specialist Clinic (IESC), Kuliyyah of Medicine, Kuantan, Pahang, Malaysia. SBNP and KCD were measured using in-vivo confocal microscopy (IVCM).
Instrument Setting
In this study, in-vivo confocal microscopy [Hiedelberg Retinal Tomograph III Rostock Cornea Module (HRT III RCM); Heidelberg Engineering GmbH, Heidelberg, Germany). The IVCM illumination utilized a 670nm diode Class 1 laser system and hence not posed any safety hazard. An 63X objective lens with a numerical aperture of 0.9 and a close working distance relative to the applanating TomocapTM (Heidelberg Engineering GmbH, Heidelberg, Germany) was used in this study. IVCM field of measurement was 400x400µm with a 1µm laser beam spot. The optical resolution/pixel for the 2D digital image size produced was 384x384 pixels. Live imaging of the cornea by a charge-coupled device (CCD) camera attached to the microscope enables the examiner to determine the exact location of the examination (Figure 1). All scans were conducted on the central region of the cornea at the level of the stroma layer and sub-basal nerve plexus by a single examiner. A drop of topical anaesthetic 0.5% proparacaine hydrochloride ophthalmic solution (AlcaineTM, Alcon Laboratories, Inc., Fort Worth, TX) was instilled in participants' eyes. A carbomer gel (GentealTM Gel; Alcon Pharmaceuticals Ltd., Switzerland) was applied onto and over the objective tip as an immersion fluid, to prevent any cornea abrasion.
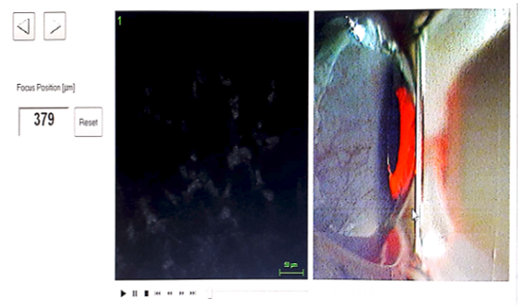
Figure 1:Live imaging of the cornea by a CCD camera.
Keratocytes Cell Density (KCD) Evaluation: Thickness Measurement
Stromal layers is defined as the distance between the first focused image of the most anterior keratocytes and the last focused image of the posterior keratocytes without images of endothelial cells. In this study, stromal thickness were divided into five (5) layers. The five subdivided stroma layers are; anterior (0% to 10%), middle (11% to 33%; 34% to 66%) and posterior (67% to 90%; 91% to 100%) as depicted in Figure 2 [27].
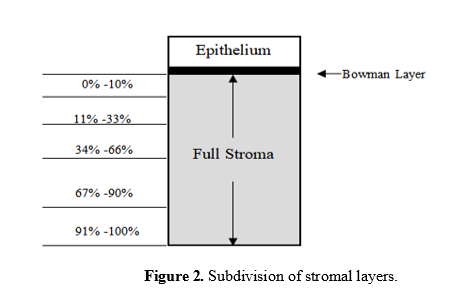
Figure 2:Subdivision of stromal layers.
Keratocyte Cell Density (KCD) Measurement
KCD was determined by manually counting the keratocyte nuclei (bright objects) in the selected confocal images within a predetermined region of interest (ROI) set as ROI: 0.16033585mm2 based on the tip size of the cap as shown in Figure 3. The image was captured and processed using ImageJTM Software (ImageJTM, US National Institutes of Health, Bethesda, Maryland, USA) in form of Joint Photographic Experts Group (JPEG) files [27-30].
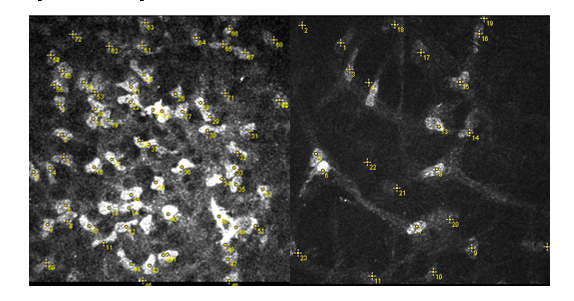
Figure 3:Anterior stroma KCD measurement (Left: anterior stroma, right: posterior stroma).
Sub-Basal Nerve Plexus (SBNP) Measurement
SBNP was manually traced in each image within the area of interest, ROI equivalent to 0.16033585mm2, using Neuron J plugin with ImageJTM Software (ImageJTM, US National Institutes of Health, Bethesda, Maryland, USA) [27-31] as shown in Figure 4. Each nerve analysis was based on several definitions [32,33]; a.) Sub-basal nerve density, NFD (mm/mm2): was defined as the total length of all visible sub-basal nerves in a frame divided by the frame area (400x400µm), b.) Nerve branch Density, NBD: The total number of main nerve branches divided by the area of the frame, and c.) Total Nerve length, NFL: Total length of visible sub-basal nerves in a frame.
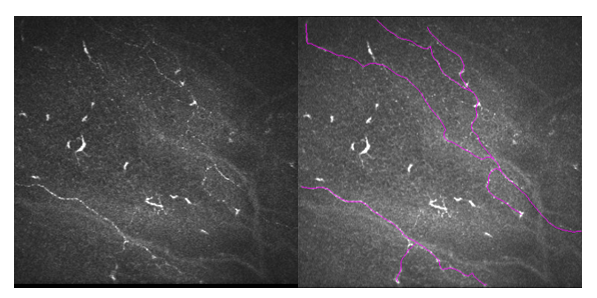
Figure 4:SBNP tracing using Neuron J in image captured in ImageJ.
Image Selection
All image acquisitions were done in standardized protocol as previously described [34]. In this study, only central cornea images were selected for analysis. To ensure this, the red reflex of the laser beam was required to be exactly in the centre of the eye in the live image. Thus, vertical nerve orientation would indicates central cornea, while oblique orientation indicates a peripheral cornea. And only high-quality images with paucity pressure of lines, optimal contrast, and absence of overlapping in either from epithelial or stromal layers.
Statistical Analysis
In this study were in this study, all images were double-blind sorted randomly using randomization software (Research Randomizer, Version 4.0, downloaded from www.randomizer.org). Two observers (NSMR and MRH) were masked in terms of sequence and all participants identifications for each image were de-identified. The average of three measurements from each image were taken for SBNP and KCD analyses. All data were expressed in mean and standard deviation. Normality testing was based on ratio of skewness kurtosis with ± 2.50 are considered as normally distributed [12]. Statistical analyses were divided into two parts; descriptive and reliability testing.
For descriptive statistics, Paired T-test was employed to evaluate test-retest repeatability, and independent T-test was done to evaluate reproducibility for measurement of stromal layer thickness. For reliability and validity testing of SBNP and KCD measurement, Intraclass correlation coefficient (ICC) and was used. ICC was employed to evaluate intra and inter-observer repeatability and reproducibility respectively. Excellent ICC was considered if the value is more than 0.75. For inter-observer, a minimum one-week time interval was set for both observers. All statistical analyses were performed using IBM SPSS (Predictive analytics software) Version 24 (IBM Corp, Armonk, NY, USA). P < 0.05 was set as the level of significance.
Results
The mean age of participants was 25.2 ± 64.8 years (N = 120 eyes). Normality testing showed all data were normally distributed (p > 0.05). Based on descriptive analysis, both observers findings showed no significance difference intra and inter-observer (both P-value > 0.05) in measurement of stromal layer, as summarized in Table 1.
Reliability of KCD measurement
This study found that reliability of KCD measurements were excellent across all statistical tests done which includes descriptive analysis and intraclass correlation coefficient (ICC). Descriptive analysis showed both observers were comparable intra and inter-observer (both P-value > 0.05) in measurement of KCD across all stromal layer. In addition, ICC also revealed excellent correlation between intra and inter-observer with all ICC was more than 0.99, as summarized in Table 1 below.
Table 1: Descriptive and correlation analyses for KCD.
| Keratocyte Cell Density (KCD) | ||||||
|---|---|---|---|---|---|---|
| Stroma Area | Layer (%) | Observer 1 | Observer 2 | P-value* | Reliability Test | |
| Mean ± SD | Mean ± SD | ICC# | Range | |||
| Anterior | 0 -10 | 518.78 ± 71.56 | 518.07 ± 70.94 | 0.197 | 0.999 | 0.997 - 0.999 |
| Middle | 11-33 | 255.87 ± 33.60 | 255.46 ± 34.07 | 0.132 | 0.998 | 0.996 - 0.999 |
| 34-66 | 196.29 ± 28.14 | 196.79 ± 28.05 | 0.185 | 0.995 | 0.991 - 0.997 | |
| Posterior | 67-90 | 183.81 ± 24.46 | 183.66 ± 24.76 | 0.596 | 0.961 | 0.928 - 0.979 |
| 91-100 | 171.74 ± 24.90 | 171.44 ± 25.29 | 0.445 | 0.998 | 0.996 - 0.999 | |
*Independent T-test
#ICC: Intraclass correlation
Reliability of SBNP measurement
Based on statistical findings, this study found that reliability of SBNP measurements were excellent across all statistical tests done which includes descriptive analysis and intraclass correlation coefficient (ICC). Descriptive analysis showed both observers were comparable intra and inter-observer (both P-value > 0.05) in measurement of KCD across all stromal layer. In addition, ICC also revealed excellent correlation between intra and inter-observer with all ICC was more than 0.99, as summarized in Table 2 below.
Table 2: Descriptive and correlation analyses for SBNP.
| Sub-basal Nerve Plexus (SBNP) | |||||
|---|---|---|---|---|---|
| Corneal nerve parameters | Observer 1 | Observer 2 | P-value* | Reliability Test | |
| Mean ± SD | Mean ± SD | ICC# | Range | ||
| NFD (number/mm2) | 29.26 ± 4.32 | 29.16 ± 4.71 | 0.323 | 0.995 | 0.990 - 0.997 |
| NBD (number/mm2) | 27.29 ± 15.64 | 27.99 ± 15.26 | 0.126 | 0.998 | 0.996 - 0.999 |
| NFL (mm/mm2) | 9127.93 ± 1830.59 | 9213.22 ± 1842.83 | 0.307 | 0.979 | 0.961 - 0.989 |
*Independent T-test
#ICC: Intraclass correlation
NFD: Nerve fiber density
NBD: Nerve branch density
NFL: Nerve fiber length
Discussion
This study aimed to evaluate validity measurement of both SBNP and KCD, with stromal thickness as reference. In-vivo confocal microscopy (IVCM) has been used in clinical application since 25 years ago, and able to provides 4-dimensional high-resolution images of corneal cells and structures. IVCM has been used in many clinical applications such monitoring cellular changes in corneal wound healing [35], endothelial keratoplasty [36], pre- and post-corneal crosslinking procedures [37] and laser refractive surgeries [38]. On top of that, IVCM also has, and current been used to evaluate effects of contact lens wear on the corneal morphology and corneal epithelial homeostasis [39].
This current study found that IVCM are reliable and valid in measuring SBNP and KCD in corneal wound healing patient particularly in post laser refractive surgery patients. Many studies [40-42] had commented that measurement of SBNP and KCD using image analysis were reliable. SBNP and KCD are important factors in determining visual performance or quality after laser refractive surgery as symptoms of dry eye [43, 44] and increase in aberration [45,46] are common. Thus, at cellular level, both SBNP and KCD could be used as early indicator for corneal regeneration or corneal wound healing which represent possibility of future development of dry eye and other ocular surface disorders.
This study would like to highlight several potential advantages and disadvantages or errors in measuring both SBNP and KCD. This method provides several benefits, firstly this method able to measure the actual corneal nerve fibres branch tortuosity, length and density accurately. Also, secondly, this method is easy to implement and less time required. In contrast, several disadvantages should be highlighted. Firstly, the current method may incorrectly identify the depth of corneal nerve measured due to its background contrast of the cornea and iris. The illumination variability of each digital image also might cause the method to incorrectly measure the corneal nerve parameters which gives rise to its reliability. However, we have minimized this error by implementing several approaches such as excluding poor quality images. Secondly, the measurement of each designated corneal stromal area were set approximately similar across all participants based on the calibration. Thus, the scanning depth were approximately equal in all eyes. This issue has been addressed by repeating the measurement using
A second grader whose result has shown a good agreement. More studies are needed to explore the effect of laser refractive surgery on the ocualr surface such as post-LASIK dry eye.
Conclusion
Measurement of corneal stromal thickness, SBNP and KCD using IVCM is valid and reliable.
Acknowledgement: This study was funded by Ministry of Higher Education Malaysia under Fundamental Research Grant Scheme 2019 (FRGS/1/2019/SKK06/UIAM/02/12)
References
- Morgan IG, Ohno-Matsui K, Saw SM. (2012) Myopia. Lancet (London England). 379 (9827): 1739-1748. [PubMed.]
- Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, et al. (2016) Global variations and time trends in the prevalence of childhood myopia a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. British J ophthalmol. 100(7): 882-890. [PubMed.]
- Czepita D. (2014) Myopia - incidence pathogenesis management and new possibilities of treatment. Russian Ophthalmological Journal. 7: 96-101. [Ref.]
- Hashemi H, Fotouhi A, Yekta A, Pakzad R, Ostadimoghaddam H, et al. (2017) Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J Curr Ophthalmol. 30(1): 3-22. [PubMed.]
- Pesudovs K, Garamendi E, Elliott DB. (2006) A quality of life comparison of people wearing spectacles or contact lenses or having undergone refractive surgery. J Refract Surg. 22(1): 19-27. [PubMed.]
- Lamoureux EL, Wang J, Aung T, Saw SM, Wong TY. (2008) Myopia and Quality of Life: The Singapore Malay Eye Study (SiMES). Invest Ophthalmol Vis Sci. 49(13): 4469. [Ref.]
- Karimian F, Feizi S, Doozande A. (2010) Higher-order aberrations in myopic eyes. J Ophthalmic Vis Res. 5(1): 3-9. [Ref.]
- Kandel H, Khadka J, Goggin M, Pesudovs K. (2017) Patient-reported Outcomes for Assessment of Quality of Life in Refractive Error: A Systematic Review. Optom Vis Sci. 94(12): 1102-1119. [PubMed.]
- Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. (2019) Corneal nerves in health and disease. Prog Retin Eye Res. 73: 100762. [PubMed.]
- Dermer H, Hwang J, Mittal R, Cohen AK, Galor A. (2021) Corneal sub-basal nerve plexus microneuromas in individuals with and without dry eye. Br J Ophthalmol. : bjophthalmol. 2020-317891. [PubMed.]
- Shehadeh-Mashor R, Mimouni M, Shapira Y, Sela T, Munzer G, et al. (2019) Risk Factors for Dry Eye After Refractive Surgery. Cornea. 38(12): 1495-1499. [PubMed.]
- Downie LE, Golborne CN, Chen M, Ho N, Hoac C, et al. (2018) Recovery of the sub-basal nerve plexus and superficial nerve terminals after corneal epithelial injury in mice. Exp Eye Res. 171: 92-100. [PubMed.]
- Arif FAC, Hilmi MR, Kamal MK, Ithnin MH. (2020) Evaluation of 18 Artificial Tears Based on Viscosity and pH Malaysian J Ophthalmol. 2(2): 96 - 111. [Ref.]
- Arif FAC, Hilmi MR, Kamal MK, Ithnin MH. (2021). Comparison of Immediate Effects on Usage of Dual Polymer Artificial Tears on Changes in Tear Film Characteristics Malaysian J Med Health Sci (MJMHS). 17(3): 252-258. [Ref.]
- Md Radzi H, Kamal MK, Che Azemin MZ. (2022) Clinical Features of Lid Margin Meibomian Gland and Tear Film Changes in Patients with Primary Pterygium. J Ophthalmic Res and Ocular Care In press. [Ref.]
- Md Radzi H, Azemin MZC, Kamal MK, Md Tamrin MI, Gaffur NA, et al. (2017) Prediction of changes in visual acuity and contrast sensitivity function by tissue redness after pterygium surgery. Curr Eye Res. 42(6): 852-856. [PubMed.]
- Md Radzi H, Kamal MK, Azemin MZC, Azami MH, Ariffin AE. (2018) Measurement of contrast sensitivity using the M&S smart system II compared with the standard Pelli-Robson chart in patients with primary pterygium. Makara J Health Res. 22(3): 167-171. [Ref.]
- Md Radzi H, Norazmar NA, Kamal MK, Che Azemin, MZ Maruziki NN, Musa NH et al. (2019) Comparison of visual acuity and contrast sensitivity between unilateral primary Pterygium and normal adults utilizing computerized M&S smart system II. International Journal of Allied Health Sciences (IJAHS). 3(2): 643-648. [Ref.]
- Md Radzi, H Maruziki NN, Kamal MK, Azemin MZC, Norazmar NA, et al. (2019) Topographic Changes as Predictor for Determining Anterior Corneal Curvature Stabilization Point Subsequent to Pterygium Excision Using Controlled Partial Avulsion Fibrin Glue Technique. International Journal Allied Health Sciences (IJAHS). 3(2): 734-740. [Ref.]
- Md Radzi H, Musa NH, Kamal MK, Azemin MZ, Maruziki NN, et al. (2019) Changes in apical corneal curvature in unilateral primary Pterygium and normal adults using simulated-K and corneal irregularity measurement. International Journal of Allied Health Sciences (IJAHS). 3(2): 588-594. [Ref.]
- Md Radzi H, Kamal MK, Azemin MZ, Ariffin AE. (2019) Corneo-pterygium total area measurements utilising image analysis method. J Optom. 12(4): 272-277. [PubMed.]
- Che Rosli NS, Md Radzi H, Kamal MK, Md Mustafa MMS. (2020) Association of net pterygium tissue mass (dry weight) in determining changes in oculovisual functions and anterior corneal curvature relative to pterygium types. International Journal of Allied Health Sciences (IJAHS). 4(1): 1042-1048. [Ref.]
- Md Radzi H, Kamal MK, Ariffin AE, Norazmar NA, Maruziki NN, et al. (2020) Effects of different types of primary pterygium on changes in oculovisual function. Sains Malaysiana. 49(2): 383-388. [Ref.]
- Cook WH, McKelvie J, Wallace HB, Misra ST. (2019) Comparison of higher order wavefront aberrations with four aberrometers. Indian J Ophthalmol. 67(7): 1030-1035. [PubMed.]
- Moshirfar M, Motlagh MN, Murri MS, Momeni-Moghaddam H, Ronquillo YC, et al. (2019) Galilei Corneal Tomography for Screening of Refractive Surgery Candidates: A Review of the Literature Part II. Med Hypothesis Discov Innov Ophthalmol. 28(3): 204-218. [PubMed.]
- Xu Y Deng J, Zhang B, Xu X, Cheng T, Wang J, et al. (2022). Higher-order aberrations and their association with axial elongation in highly myopic children and adolescents. Br J Ophthalmol. 319769. [PubMed.]
- Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. (2003) Keratocyte density in the human cornea after photorefractive keratectomy. Arch Ophthalmol. 121(6): 770-776. [PubMed.]
- Rasband WS, ImageJ U. S. National Institutes of Health Bethesda Maryland USA. 1997-2018. [Ref.]
- Jordan C, Patel DV, Abeysekera N, McGhee CN. (2014) In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross-linking in keratoconus. Ophthalmology. 121: 469-474. [PubMed.]
- Patel SV, Erie JC, McLaren JW, Bourne WM. (2007) Confocal microscopy changes in epithelial and stromal thickness up to 7 years after LASIK and photorefractive keratectomy for myopia. J Refract Surg. 23(4): 385-392. [PubMed.]
- Cottrell P, Ahmed S, James C, Hodson J, McDonnell PJ, et al. (2014) Neuron J is a Rapid and Reliable Open-Source Tool for Evaluating Corneal Nerve Density in Herpes Simplex Keratitis. Invest. Ophthalmol. Vis. Sci. 55(11): 7312-7320. [PubMed.]
- Mohamed-Noriega K, Riau AK, Lwin NC, Chaurasia SS, Tan DT, et al. (2014) Early Corneal Nerve Damage and Recovery Following Small Incision Lenticule Extraction (SMILE) and Laser in Situ Keratomileusis (LASIK). Invest. Ophthalmol. Vis. Sci. 55(3): 1823-1834. [PubMed.]
- Oliveira-Soto L, Efron N. (2001) Morphology of corneal nerves using confocal microscopy. Cornea. 20(4): 374-384. [PubMed.]
- Kalteniece A, Ferdousi M, Adam S, Schofield J, Azmi S, et al. (2017) Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One. 12(8): e0183040. [PubMed.]
- Petroll WM, Robertson DM. (2015) In Vivo Confocal Microscopy of the Cornea: New Developments in Image Acquisition Reconstruction and Analysis Using the HRT-Rostock Corneal Module. Ocul Surf. 13(3): 187-203. [PubMed.]
- Kobayashi A, Mawatari Y, Yokogawa H, Sugiyama K. (2008) In vivo laser confocal microscopy after Descemet stripping with automated endothelial keratoplasty. Am J Ophthalmol. 145: 977-985. [PubMed.]
- Bouheraoua N, Jouve L, El Sanharawi M, Sandali O, Temstet C, et al. (2014) Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 55: 7601-7609. [Ref.]
- Mohebbi M, Rafat-Nejad A, Mohammadi SF, Asna-Ashari K, Kasiri M, et al. (2017) Post-photorefractive Keratectomy Pain and Corneal Sub-basal Nerve Density. J Ophthalmic Vis Res. 12(2): 151-155. [PubMed.]
- Cardigos J, Barcelos F, Carvalho H, Hipólito D, Crisóstomo S, et al. (2019) Tear Meniscus and Corneal Sub-basal Nerve Plexus Assessment in Primary Sjögren Syndrome and Sicca Syndrome Patients. Cornea. 38(2): 221-228. [PubMed.]
- Sterenczak KA, Winter K, Sperlich K, Stahnke T, Linke S, et al. (2021) Morphological characterization of the human corneal epithelium by in vivo confocal laser scanning microscopy. Quant Imaging Med Surg. 11(5): 1737-1750. [PubMed.]
- Bullet J, Gaujoux T, Borderie V, Bloch I, Laroche L. (2014) A reproducible automated segmentation algorithm for corneal epithelium cell images from in vivo laser scanning confocal microscopy. Acta Ophthalmol. 92(4): e312-316. [PubMed.]
- Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo, et al. (2018) Neurotrophic keratopathy. Prog Retin Eye Res. 66: 107-131. [PubMed.]
- Liu Q, Xu Z, Xu Y, Zhang J, Li Y, et al. (2020) Changes in Corneal Dendritic Cell and Sub-basal Nerve in Long-Term Contact Lens Wearers with Dry Eye. Eye Contact Lens. 46(4): 238-244. [PubMed.]
- Cruzat A, Pavan-Langston D, Hamrah P. (2010) In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 25(5-6): 171-177. [PubMed.]
- Li L, Cheng GPM, Ng ALK, Chan TCY, Jhanji V, et al. (2017) Influence of Refractive Status on the Higher-Order Aberration Pattern After Small Incision Lenticule Extraction Surgery. Cornea. 36(8): 967-972. [PubMed.]
- Recchioni A, Sisó-Fuertes I, Hartwig A, Hamid A, Shortt AJ, et al. (2020) Short-Term Impact of FS-LASIK and SMILE on Dry Eye Metrics and Corneal Nerve Morphology. Cornea. 39(7): 851-857. [PubMed.]