>Corresponding Author : Sergio Munoz
>Article Type : Case Report
>Volume : 5 | Issue : 3
>Received Date : 24 Oct, 2025
>Accepted Date : 03 Nov, 2025
>Published Date : 07 Nov, 2025
>DOI : https://doi.org/10.54289/JDOE2500111
>Citation : Munoz S, Shapiro S, Owens WH, Redmond DL, and Johnson TM. (2025) Bone-level Zirconia Implant Fracture in a Nickel-allergic Patient: a case report. J Dent Oral Epidemiol 5(3): doi https://doi.org/10.54289/JDOE2500111
>Copyright : © 2025 Munoz S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Department of Periodontics, Saunders Dental Clinic, Keller Army Community Hospital (KACH), West Point, New York
2Department of Oral and Maxillofacial Surgery, Multi-Specialty Clinic, Keller Army Community Hospital (KACH), West Point, New York
3Department of Prosthodontics, Saunders Dental Clinic, Keller Army Community Hospital (KACH), West Point, New York
4Department of Comprehensive Dentistry, Dental Services United States Military Academy, Keller Army Community Hospital (KACH), West Point, New York
5Department of Periodontics, Army Postgraduate Dental School, Uniform Services University of the Health Sciences, Fort Gordon, Georgia
*Corresponding author: Sergio Munoz, Department of Periodontics, Saunders Dental Clinic, Keller Army Community Hospital (KACH), West Point, New York
Abbreviations: RPD: Removable Partial Dentures, FDP: Fixed Dental Prosthesis, CC: Chief Concern, GERD: Gastroesophageal Reflux Disease, EIA: Exercise-Induced Asthma, PAR: Perennial Allergic Rhinitis, ARP: Alveolar Ridge Preservation, LWSA: Lateral Window Maxillary Sinus Floor Augmentation, FCLP: Functional Crown Lengthening Procedure, rpm: Revolutions Per Minute, N·cm: Newtons-Centimeters, PEEK: Polyetheretherketone, IFU: Instructions For Use, PTFE: Polytetrafluoroethylene, CP: Commercially Pure, Ti: Titanium, PPM: Parts Per Million, LTD: Low-Temperature Degradation, XRD: X-Ray Diffraction, FIB: Focused Ion Beam
Introduction
Despite the advances in dentistry, single tooth replacement is limited to two broad treatment modalities: removable and fixed dental prostheses. On the one hand, it is hypothetically possible for removable partial dentures (RPDs) to replace single teeth defects. However, in practice, there are at least three relative contraindications precluding their use. First, RPDs encourage bioburden build up resulting in plaque index, gingival index, calculus index, and probing depth increase [1]. Second, studies have shown that RPDs increase the chances for dental caries. In a prospective randomized clinical trial, patients that wore RPDs had a 60% greater incidence of new and recurrent carious lesions [2]. Third, RPDs can present with significant fabrication defects. The high prevalence of defects to include base material cracks and holes, missing or chipped denture teeth, broken or distorted clasps and rests result in the lack of intraoral stability [3] and function which contributes to patient dissatisfaction.
On the other hand, it makes more sense to replace a single missing tooth with a fixed dental prosthesis (FDP) which can be secured to teeth or implants [4]. The most practical option for a bounded edentulous space is a fixed partial denture (FPD) which employes two natural teeth as abutments for anchorage and an artificial tooth known as a pontic to restore the missing tooth [4]. Another practical option involves the surgical placement of a single implant which is allowed to osseointegrate and later restored with a screw or cement retained crown thus replacing the missing single tooth.
When deciding on the most appropriate treatment option for a single tooth replacement, the dentist and the patient need to talk about several issues that will help determine the best treatment choice. The topics of discussion include morbidity of the treatment, durability of the prosthesis, esthetics of the final restoration, cost, and patient desires. If there are no absolute contraindications, the patient’s desires driven by his/her expectations and preferences may very well determine the treatment modality of choice. The purpose of this case report is to present a patient that wants to replace missing tooth #14 with an implant and implant supported restoration despite having a nickel allergy.
Material and Methods
Clinical presentation
On June 3, 2024, a 50-year-old female patient presents to the Keller Army Community Hospital Dental Clinic Periodontics Department at West Point New York with a chief concern (CC): “the prosthodontist told me that I needed surgery to receive a crown on my lower left tooth. I am also missing a tooth on the upper left and I would like an implant for that gap.” Her medical history is significant for gastroesophageal reflux disease (GERD), exercise-induced asthma (EIA), and perennial allergic rhinitis (PAR). She takes cetirizine, pantoprazole, and montelukast every day, but levalbuterol as needed. Review of her medical chart confirms allergies to nickel, lanolin, and sulfa drugs. Nickel and lanolin allergies were diagnosed using a skin patch test. During her initial evaluation, she made it clear that she has irrational dental anxiety and worse yet, surgical phobia. She has a Mallampati class 4 airway. She has been diagnosed with nocturnal bruxism and wears a maxillary hard night guard to mitigate occlusal wear. The tooth on the maxillary left side is missing because it fractured below the alveolar crest and the oral surgeon had to take the patient to the OR to extract tooth #14 and performed an alveolar ridge preservation (ARP). In addition to the ARP, the oral surgeon performed a left lateral window maxillary sinus floor augmentation (LWSA) due to sinus pneumatization (Fig 1).
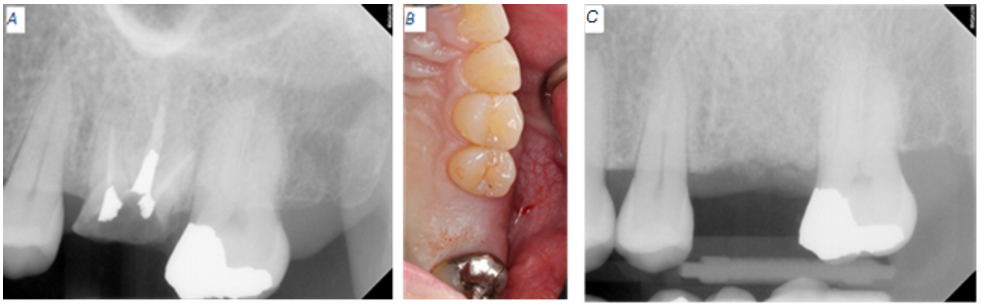
Figure 1: Baseline radiographic clinical appearance for site #14. (A) Catastrophic tooth fracture. (B) Four months after extraction of tooth #14. (C) Remaining allograft present at the extraction site.
Concerning the mandibular left side, tooth #18 presented with recurrent caries around a composite restoration that replaced the distolingual cusp. The distal margin of this composite projects apically beyond the cavity preparation and appeared to approach the crestal bone (Fig 2).
Based on the radiographic and clinical findings, the following diagnosis applied to site #14, acquired partial edentulism. As for tooth #18, the following diagnoses applied, recurrent caries, short clinical crown, distal composite overhang, and restorative margin within the supracrestal attached tissue. The proposed surgical treatment plan included the placement of a zirconia implant at site #14 and functional crown lengthening procedure (FCLP) for tooth #18.
Case management
After the initial patient evaluation and prior to taking the patient to the OR, a consultation with the oral surgeon and the prosthodontist took place to make sure that all materials, supplies, and equipment were on hand for the placement and restoration of a zirconia implant at site #14. Given the documented nickel allergy, we decided to consult with a few major implant manufacturers such as Straumann, Nobel Biocare, ZimVie, and Zeramex to see if a zirconia implant or another 100% metal-free solution was available. Given that we are one of the three military service dental providers, we were quickly reminded that we had access to only two implant manufacturers: ZimVie and Nobel Biocare. ZimVie does not make any ceramic implants; therefore, Nobel Biocare, maker of the two-piece alumina-toughened zirconia implant, became our final choice [5].
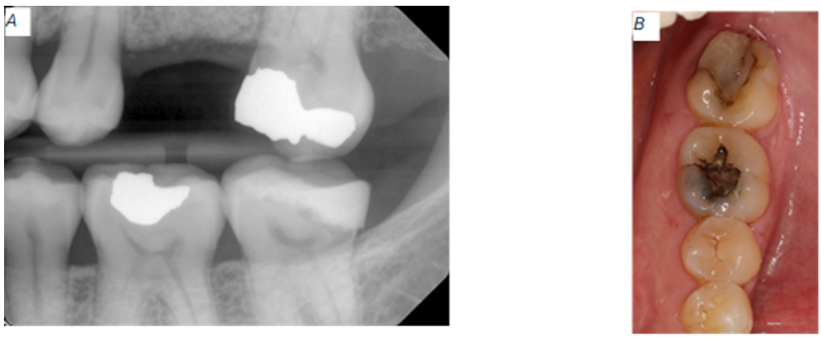
Figure 2: Baseline radiographic and clinical appearance for tooth #18. (A) Excess restorative material nearing the alveolar bone. (B) Recurrent caries around the composite restoration.
In the OR on the day of surgery, the surgical team performed a FCLP for tooth #18 and placed a NobelPearl implant at site #14. For the implant placement, we elevated a full mucoperiosteal flap extending from tooth #13 to tooth #15. Sequential osteotomy was done using the NobelPearl Round Bur 2 mm, Tapered Twist Drill 2.3 mm, Tapered Drill NP 3.5 x 10 mm, Tapered Drill RP 4.2 x 10 mm, and Screw Tap RP 4.2 mm. A NobelPearl Tapered 4.2 x 10 mm RP implant was placed at a crestal level using the NobelPearl Implant Driver Inter-X at a speed of 15 revolutions per minute (rpm). The implant was tightened with the manual torque wrench to 30 Newtons-centimeters (N·cm) (Fig 3). A NobelPearl Polyetheretherketone (PEEK) Healing Abutment was place on the implant and torqued to 5 N·cm. All the implant placement steps, tooling, speeds, torque, and irrigation requirements were followed as depicted in the NobelPearlTM procedures manual and instructions for use (IFU) [6,7].
Results
Two weeks post-implantation
After two weeks of healing, our patient presented for post-operation follow-up. During this appointment, we obtained a new periapical radiograph for site #14 and performed a clinical evaluation. The patient didn’t have any concerns, and she didn’t report any complications. Clinically and radiographically, everything was healing within normal limits and sutures remained intact. The tissues surrounding site #14 and tooth #18 were superficially debrided with a cotton tip applicator saturated with 0.12% chlorhexidine gluconate and the remaining gut sutures were removed. The patient received a post-operation brush to restart mechanical oral hygiene on and around the surgical sites (Fig 4).
Six months post implant placement
At the six months appointment, the PEEK healing abutment was removed from the osseointegrated implant as site #14. A NobelPearl Straight Engaging Inter-X Prefabricated Zirconia Abutment was tried and torque to only 15 N·cm to allow for occlusal reduction until enough space was available for the final restoration. After abutment modification, the abutment was torqued to 25 N·cm using the Vicarbo NobelPearl Definitive Clinical Screw Inter-X (carbon-fiber reinforced PEEK). The access screw channel was obturated using Polytetrafluoroethylene (PTFE) tape. An IPS e.max crown was fabricated using the integrated CEREC Dental CAD/CAM System. The e.max crown was cemented using Rely-X Luting (resin modified glass ionomer cement). Excess luting cement was removed, and a verification periapical radiograph was obtained (Fig 5). Twelve days after the implant supported, cement retained restoration for site #14 was delivered, a flat plane, full arch, hard night guard was delivered to decrease the chances of biomechanical overloading due to excessive and unintentional occlusal force on the implant restoration.
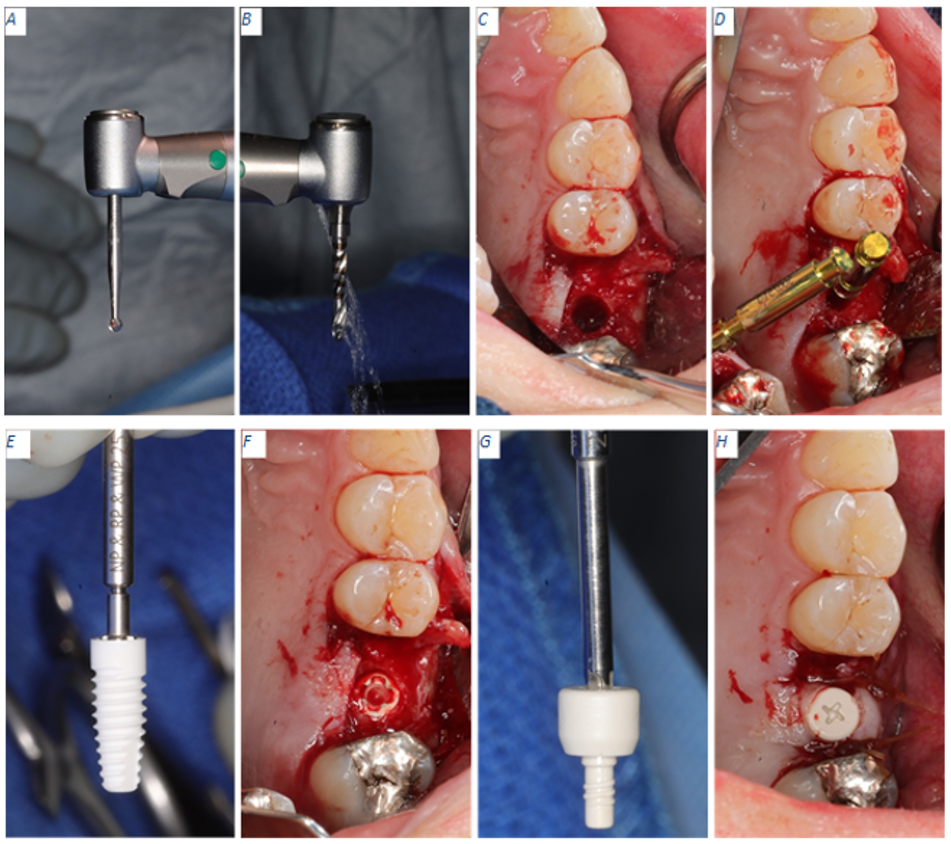
Figure 3: Implant placement workflow. (A) NobelPearl Round Bur 2 mm used to create an initial pilot hole for freehand implant placement protocol. (B) NobelPearl Tapered Twist Drill 2.3 mm running at a maximum of 800 rpm to form the initial osteotomy. (C) Final osteotomy completed with the NobelPearl Tapered Drill RP 4.2 x 10 mm running at a maximum of 600 rpm. (D) NobelPearl 4.2 mm Depth/Direction indicator in place. (E) Implant placement with NobelPearl Implant Driver Inter-X. (F) NobelPearl Implant in osteotomy at crestal level. (G) NobelPearl Healing Abutment ready for insertion using the NobelPearl Screwdriver. (H) Healing abutment torqued to 5 N·cm.
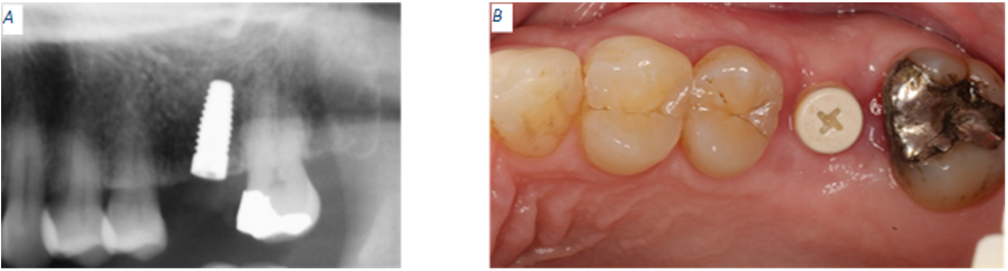
Figure 4: Two weeks post operation. (A) PEEK abutment not visible in periapical radiograph. Implant appears to have a distal tilt. (B) Site #14 displays soft tissue healing within normal limits.
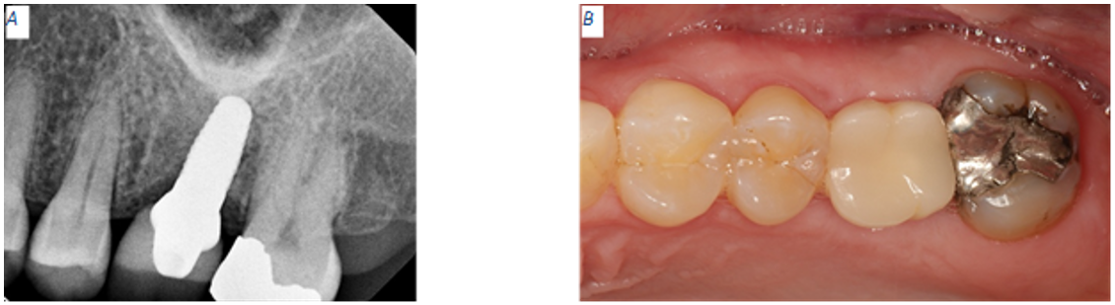
Figure 5: Two weeks post operation. (A) PEEK abutment not visible in periapical radiograph. Implant appears to have a distal tilt. (B) Site #14 displays soft tissue healing within normal limits
Four months after receiving final implant crown
Patient presents for an emergency dental visit four months after the implant supported, cement retained crown was delivered for site #14. The patient’s CC is that she feels that her implant is loose, but she does not have any pain or bleeding. Patient reports that a few weeks ago she heard a crunch while eating a breakfast sandwich but there was no apparent mobility or pain at that time. Intraoral evaluation reveals mobility of the implant crown. Periapical radiograph confirms a catastrophic implant fracture (Fig 6).
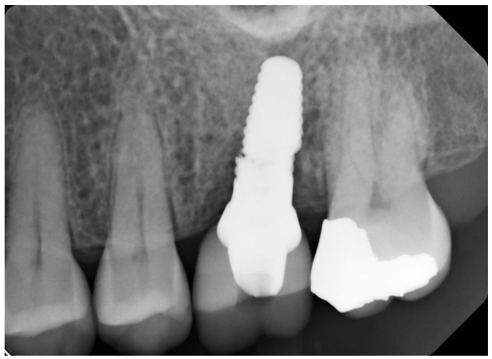
Figure 6: Abrupt fracture of zirconia implant.
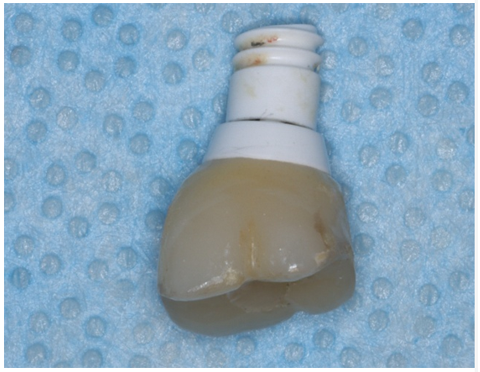
Figure 7: Patient retrieved piece of implant attached to crown.
The prosthodontist offered to remove the broken implant piece to reduce the chances of swallowing or aspirating the loose part, but the patient refused. She agreed to consult with the oral surgeon and settled into going back to the OR to have the loose implant piece removed and receive a bone graft to cover the osseointegrated apical piece while at the same time reducing the chance of developing a major crestal ridge defect. The patient and the prosthodontist discussed the possibility of replacing missing tooth #14 with a three-unit FPD, but the patient refused this kind of treatment. A few days later, the patient returned to the dental clinic with the coronal portion of the fracture implant and the implant crown in her hand (Fig 7).
Discussion
The patient featured in this case study suffers from severe dental anxiety; she is specifically fearful of dental procedures (odontophobia). Dental anxiety seems to affect many people, but the occurrence reports vary depending on the study design, country of origin, and cultural factors. In a systematic literature review and meta-analysis that included population-based studies from many countries, reported that the global prevalence of dental anxiety is 15.3% [8]. However, in a cross-sectional study aimed at U.S. adults, 72.6% of the study population reported to have moderate dental fear while 26.8% reported to have severe dental fear [9]. Furthermore, dental anxiety prevalence reports may be affected by cultural factors. There is a link between negative attitudes towards basic oral care and dentistry in some cultures which translate into higher dental anxiety among the individual members of those cultures. This cultural fear spread horizontally, from person to person, and vertically from parent to child. In a meta-analysis, 79% of the appraised studies showed a relationship between parent and child dental fear [10].
This patient’s odontophobia, Mallampati class 4 airway, nickel allergy, and strong determination, forced us to treat her in an atypical manner. Firstly, all preventive, restorative, and surgical dental appointments required greater time allotment. Secondly, the patient needed the opinion of multiple specialists which resulted in additional appointments to coordinate multidisciplinary conferences. Thirdly, routine surgical procedures that are normally performed in a clinical setting had to be completed in the OR. Finally, the multidisciplinary team had to place a zirconia implant where we normally place a titanium one. There are two reasons why we chose to place a zirconia implant. First, our patient was determined to have an implant rather than an FPD. Second, the patient has a medically recognized nickel allergy. Nickel allergies have been well documented in the literature and as such, researchers have concluded that nickel is the most common metal sensitizer in humans. Immunologists in the United States, Finland, Denmark, Spain, Sweden, Canada, and Norway have patch tested for nickel allergy with a total positive reaction that ranged from as low as 3.9% to as high as 20% of the corresponding study population [11]. Overall, the average occurrence of skin nickel hypersensitivity in the general population is 13-14% [12]. Given the high nickel allergy prevalence, we could not treatment plan a titanium implant. Even though Commercially Pure (CP) Titanium (Ti) Grade IV and Ti alloy (Ti-6Alumium[Al]-4Vanadium[V]) are bioinert [13] and widely used materials in the fabrication of dental implants, they are known to have impurities and contaminants such as nickel, chromium, tin, arsenic, and copper. In a mass and optical emission spectrometry elemental study, nickel was found at an absolute amount of 56 parts per million (PPM) in all investigated implants [14]. Even at a small amount, nickel in CP Ti and in Ti6Al4V carry a potential for allergic reactions in patients with confirmed sensitivity. As advertised by the Nobel Biocare, the chemical inertness of zirconia allowed us to conclude that NobelPearl would not trigger any allergic reactions. Indeed, a chemical composition spectrum analysis of the Nobel Pearl implants measured 50.45% ZrO2, 43.73% Al2O3, and 5.82% Y2O3 accounting for 100% of the sampled mass [15].
As seen in the results, this patient’s zirconia implant failed due to a catastrophic fracture. This early failure goes against the survival rates reported in the literature. According to a systematic review and meta-analysis, the cumulative survival rate for zirconia implants is 95.1% after 10 years. Fracture, as a mode of implant failure, happens at a rate of 0.65% for zirconia implants which is comparable to that of titanium implants at a rate of 0.44% [16].
Zirconia implant fractures can be attributed to chemical and/or mechanical causes. A chemical reaction known as low-temperature degradation (LTD) happens to zirconia implants intraorally where water in the saliva interacts with the surface of the zirconia implant spontaneously changing its metastable tetragonal configuration to the stable monoclinic form.
LTD or aging has been blamed for impairing the mechanical properties of zirconia resulting in a rough surface, increase wear, microcracks, crack propagation, and material fracture [17]. However, aging is a limited and slow process, it is unlikely the cause for the catastrophic fracture seen on the implant featured in this manuscript particularly because it was in service for only four months, which is a very short period. In regard to aging, a study compared in-vitro accelerated aged zirconia dental implants to in-vivo aged zirconia femoral balls concluding that there is a direct linear relationship between aging time and the increase in monoclinic transformation for both types of samples [18]. The difference is that in the zirconia dental implants, the monoclinic transformation thickness reaches 0.7 µm at 11 hours of in-vitro aging. Continuous in-vitro aging up to 3100 hours didn’t increase the monoclinic transformation thickness in the dental implants demonstrating that this reaction is self-limiting [18]. Based on the monoclinic transformation thickness of in-vitro aged zirconia and the thickness seen on the in-vivo femoral balls, the investigators were able to calculate a 1:50 equivalence factor. This means that one hour of in-vitro aging is equivalent to 50 hours of in-vivo aging. The group with the in-vitro specimens aged to 3100 hours, equivalent to 17.7 years of in-vivo aging, didn’t have any negative effect on their mechanical properties proving that aging is not only slow, but it does not seem to affect the zirconia dental implant performance [18]. Another study that evaluated tetragonal to monoclinic transformation during accelerated aging using X-ray diffraction (XRD) and focused ion beam (FIB) concluded that zirconia implant strength and fatigue resistance were not affected by aging [19].
In this case study, the implant most likely fractured due to a bending moment overload. A bending moment is produced when a transverse load is applied to a beam [20]. In other words the occlusal force provided a right-angle load to the implant through the implant restoration, while the implant acted as a beam which bent under the load. The bending overload concentrated internal stress points where cracks initiated and propagated until the implant fractured. In an article by Zhang et al., bending overload resulted in zirconia implant material stress in which one side of the implant presented in tension and the other side in compression. Eventually, a crack formed on the tension side and propagated until a separation occurred [21]. The location and nature of the zirconia implant fracture vary depending on implant configuration, one-piece vs. two-piece. One the one hand, one-piece zirconia implants fractured at the endosseous portion between two threads. On the other hand, the two-piece zirconia implants can fracture in one of three fractographic behaviors; at the abutment neck, internal abutment-implant connection, or at the endosseous portion [21].
Regarding the implant featured in this article, the fracture happened at the base of the internal implant threads which receive the Nobel Pearl Vicarbo definitive clinical screw. The crack or cracks most likely had an internal origin but soon propagated radially between two implant threads and vertically towards the implant platform exposing the abutment. Based on this description and photos in figure 8, we can conclude that the implant failure fits the internal-implant connection fractographic behavior.
Conclusion
If a titanium implant cannot be used to replace a missing tooth due to a metal allergy, a single-piece zirconia implant may be a better choice. In a single-piece implant configuration, a single connection between the implant and crown makes a single-piece implant less complex. Also, in a single-piece implant, we worry about an implant fracture at the endosseous portion. On the contrary, in a two-piece zirconia implant, we must worry about an implant fracture at the abutment neck, at the internal abutment-implant connection, and at the endosseous portion.
In addition to using a single-piece implant, the following suggestions may help optimize the success of zirconia implants. First, use a wider diameter implant when the bone volume allows it. This is because wider implants provide additional zirconia thickness which may resist fracture. Second, if the patient has experienced an implant fracture, rather than considering another implant, a better choice is a 3-unit FPD. This choice works well when the missing tooth is not the most distal one. Third, the implant crown should have shallow anatomy and narrow occlusal table to minimize occlusal interference. Minimizing premature contacts due to occlusal interference is important to decrease the likelihood of bending moment overload. Last, fabricate and deliver an occlusal guard to help with occlusal load distribution and minimize stress concentration, this is particularly important on individuals known to have bruxism.
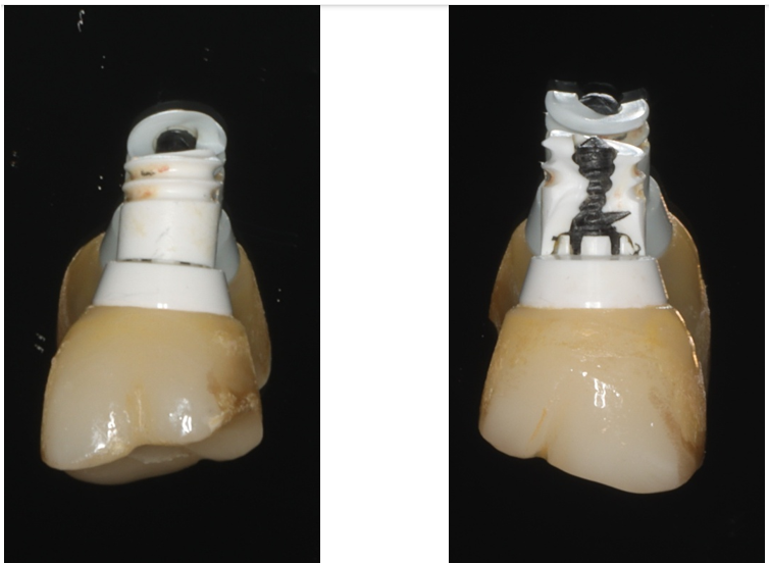
Figure 8: Internal-Implant connection fractographic behavior. (A) Implant fracture propagated radially between the 2nd and 3rd threads. (B) Vertical crack propagation exposed the Vicarbo screw and abutment.
References
- Zlataric DK., Celebic A., Valentic–Peruzovic M. The effect of removable partial dentures on periodontal health of abutment and non–abutment teeth. J Periodontol. 2002;73:137–144. [PubMed.]
- Jepson NJ., Moynihan PJ., Kelly PJ., Watson GW., Thomason JM. Caries incidence following restoration of shortened lower dental arches in a randomized controlled trial. Br Dent J. 2001;191:140–144. [PubMed.]
- Hummel SK., Wilson MA., Marker VA., Nunn ME. Quality of removable partial dentures worn by the adult U.S. population. J Prosthet Dent. 2002;88:37–43. [PubMed.]
- Glossary of Prosthodontic Terms 10th Edition. J Prosthet Dent. 2023;130:E7–E126. [PubMed.]
- Natural esthetics and soft tissue harmony. Nobel Biocare. 2025. [Ref.]
- Nobel Pearl TM Procedures manual. NobelBiocare. 2023. [Ref.]
- Nobel Pearl Tapered Dental Implant System., instructions for use (IFU). NobelBiocare. [Ref.]
- Silveira ER., Cademartori MG., Schuch HS., Armfield JA., Demarco FF. Estimated prevalence of dental fear in adults: A systematic review and meta–analysis. J Dent. 2021;108:103632. [PubMed.]
- Heyman RE., Daly KA., Aladia S., Harris SL., Roitman NA., Kim AC., et al. A census–matched survey of dental fear and fear–treatment interest in the United States. JADA. 2025;156(9):696–705. [PubMed.]
- Themessl–Huber M., Freeman R., Humphris G., MacGillivray S., Terzi N. Empirical evidence of the relationship between parental and child dental fear: a structured review and meta–analysis. International Journal of Paediatric Dentistry. 2010;20:83–101. [PubMed.]
- Thyssen JP., Linneberg A., Menne T., Johansen JD. The epidemiology of contact allergy in the general population –prevalence and main findings. Contact Dermatitis. 2007;57:287–299. [Ref.]
- Siljander BR., Chandi SK., Debbi EM., McLawhorn AS., Sculco PK., Chalmers BP. A Comparison of Clinical Outcomes After Total Knee Arthroplasty in Patients With Preoperative Nickel Allergy Receiving Cobalt Chromium or Nickel–Free Implant. The Journal of Arthroplasty. 2023;38:S194–S198. [PubMed.]
- Sykaras N., Lacopino AM., Marker VA., Triplett RG., Woody RD. Implant Materials, Designs, and Surface Topographies: Their Effect on Osseointegration. A Literature Review. Int J Oral Maxillofac Implants. 2000;15:675–690. [PubMed.]
- Stricker A., Bergfeldt T., Fretwurst T., Addison O., Schmelzeisen R., et al. Impurities in commercial titanium dental implants–A mass and optical emission spectrometry elemental analysis. Dental Materials. 2022;38:1395–1403. [PubMed.]
- Tchinda A., Lerebours A., Kouitat–Njiwa R., Bravetti P. Zirconia Dental Implants: A Closer Look at Surface Condition and Intrinsic Composition by SEM–EDX. Bioengineering. 2023;10:1102. [PubMed.]
- Mohseni P., Soufi A., Chrcanivic BR. Clinical outcomes of zirconia implants: a systematic review and meta-analysis. Clinical Oral Investigations. 2023;28:15. [PubMed.]
- Osman RB., Swain MV. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Material. 2015;8:932–958. [PubMed.]
- Gil J., Menocal JADG., Ortega EV., Bosch B., Delgado L., et al. Comparison of zirconia degradation in dental implants and femoral balls: an X–ray diffraction and nanoindentation study. Int J Implant Dent. 2021;7:103. [PubMed.]
- Sanon C., Chevalier J., Douillard T., Kohal RJ., et al. Low temperature degradation and reliability of one-piece ceramic oral implant with a porous surface. Dental Materials. 2013;29:389–397. [PubMed.]
- Dissemination of IT for the Promotion of Materials Science (DoITPoMS). Bending moments and beam curvatures. University pf Cambridge. 2024. [Ref.]
- Zhang F., Monzavi M., Li M., Cokic S., Manesh Al., et al. Fracture analysis of one/two–piece clinically failed zirconia dental implants. Dental Materials. 2022;38:1633–1647. [PubMed.]