>Corresponding Author : Mana Rao
>Article Type : Mini Review Article
>Volume : 2 | Issue : 2
>Received Date : 29 June, 2022
>Accepted Date : 11 July, 2022
>Published Date : 15 July, 2022
>DOI : https://doi.org/10.54289/JVVD2200110
>Citation : Agarwal E, Rao M. (2022) Myopericarditis and COVID-19 Vaccination. J Virol Viral Dis 2(2): doi https://doi.org/10.54289/JVVD2200110
>Copyright : © 2022 Agarwal E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mini Review Article | Open Access | Full Text
1Northern Highlands Regional High School, Allendale NJ
2Essen Medical Associates, Bronx NY
3Archcare, New York NY
*Corresponding author: Mana Rao, Essen Medical Associates, Bronx NY, Archcare, New York NY
Abbreviations: COVID-19: Coronavirus disease of 2019, SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2, EKG: Electrocardiogram, CDC: Centers for Disease Control and Prevention, cMRI: Cardiac Magnetic Resonance Imaging, US: United States, mRNA: Messenger Ribonucleic Acid, Pfizer: Pfizer-BioNTech’s BNT162B2, Moderna: Moderna’s mRNA-1273, S: Viral Spike Glycoprotein, AZ: Oxford/Astrazeneca: Vaxzevria, VAM: Vaccination Associated Myopericarditis, VAERS: Vaccine Adverse Event Reporting System
Review
Coronavirus disease of 2019, colloquially known as COVID-19, is a multisystemic infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The most common symptoms of COVID-19 include fever, myalgia, chills, cough, shortness of breath, fatigue, along with anosmia and dysgeusia. SARS-CoV-2 infection can result in simultaneous inflammation in multiple tissues including, and not limited to, pulmonary, renal, central nervous and cardiac organ systems. Cardiac involvement in COVID-19 may be asymptomatic with cardiac biomarker changes and/or electrocardiogram (EKG) changes; or symptomatic.
Symptomatic disease may involve one or more functional elements of cardiac tissue e.g., pericardium, myocardium and the conduction system. There are numerous reports of COVID-19 associated myocarditis in the literature [1-3].
The Centers for Disease Control and Prevention’s (CDC) definition of a confirmed case of myocarditis is the presence of new or worsening clinical symptoms as listed in Table 1 along with new findings on cardiac magnetic resonance imaging (cMRI) or histopathology and no other identifiable cause of the symptoms and findings [4]. Definitions of acute pericarditis and myopericarditis are also listed in Table 1.
For simplicity, the remainder of the manuscript refers to these conditions collectively as myopericarditis. The etiologies of myopericarditis include non infectious causes, such as medication-induced, autoimmune, physical trauma, and infectious causes. Those attributable to infectious agents may be incited by common viruses including influenza and SARS-CoV-2. Myopericarditis may also occur after vaccination against viral diseases such as smallpox [5]. Among viral causes, at the molecular level, whether the virus itself infects cardiac cells resulting in a direct viral insult on cellular machinery or whether an immunologic cascade leads to cellular damage remains elusive. While chest pain is the most common symptom of myopericarditis, other symptoms include fever, sore throat, dyspnea, palpitations, and fatigue.
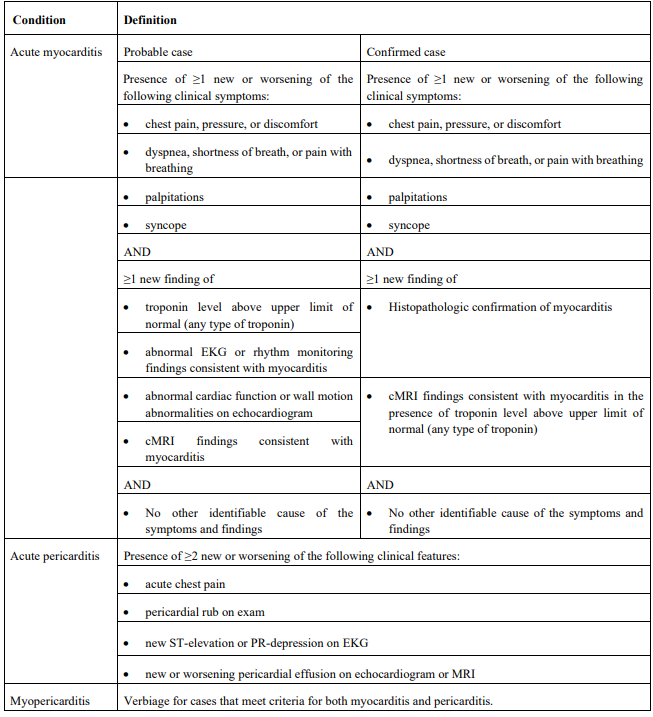
Table 1: CDC case definitions of probable and confirmed myocarditis, pericarditis, and myopericarditis [4].
The diagnostic evaluation of suspected myopericarditis should include initial laboratory testing and cardiac imaging. Initial testing should include a complete blood count, chemistries, EKG, cardiac biomarkers including serum troponin levels, chest X-ray, and nonspecific inflammatory markers such as erythrocyte sedimentation rate and C reactive protein. Cardiac imaging for myopericarditis includes an echocardiogram to evaluate for global ventricular function, valvular function, and other potential causes of cardiac dysfunction. Coronary angiography is indicated in selected patients with presentation identical to that of acute coronary syndrome. cMRI and endomyocardial biopsy are reserved for complex clinical scenarios. Following the diagnosis of viral myopericarditis, some patients experience spontaneous, gradual improvement while others require more focused care. Mild cases may be managed by conservative therapy including rest and medications, such as non steroidal anti inflammatory drugs and/or corticosteroids. Moderate to severe cases may require drugs such as, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, beta blockers, diuretics and cardiac inotropes. Lastly, intensive care is reserved for the most severe clinical presentations and treatment for these cases may need intra aortic balloon pump, extra corporeal membrane oxygenation and ventricular assist devices or heart transplant.
Vaccines against COVID-19 have been available for use in the United States (US) population since December 2020 [6]. Currently, there are four types of COVID-19 vaccines that have been listed for emergency use by the World Health Organization [7]. The first type uses messenger ribonucleic acid (mRNA) technology and includes Pfizer-BioNTech’s BNT162B2 also called Comirnaty® (Pfizer) and Moderna’s mRNA-1273 (Moderna). Nucleoside-modified mRNA encoding the viral spike glycoprotein (S) is delivered to the vaccine recipient and this ultimately results in antibody production. The second type are vector vaccines and notable manufacturers are Janssen and Oxford/AstraZeneca: Vaxzevria (AZ). S protein of SARS-CoV-2 is placed in an altered version of a viral vector and when the immune system is challenged with vaccination, robust antibody production occurs. The third type of COVID-19 vaccination consists of protein subunit vaccines, manufactured by Novavax and Serum Institute of India, the active ingredient being SARS-CoV-2 S protein. The fourth type of COVID-19 vaccine contains inactivated virus and prominent manufacturers include Bharat Biotech, Sinopharm and Sinovac. While COVID-19 vaccines have demonstrated potency and efficacy in reducing COVID-19 related hospitalizations and severe disease, they have also been implicated in vaccination associated myopericarditis (VAM).
A surveillance system in the US called ‘Vaccine Adverse Event Reporting System’ (VAERS) records instances of adverse events following immunization, including COVID-19 VAM. More than 192 million people received an mRNA vaccine from December 2020 through August 2021. Recent reports indicate that COVID-19 VAM could occur following mRNA vaccination, including Pfizer and Moderna. COVID-19 VAM cases have been observed primarily in adolescent and young adult males. The second dose of mRNA vaccines notably confer a higher risk. Oster et al reported that 1626 cases met the definition of myocarditis following COVID-19 vaccination; the majority of these cases occurred following the second dose of vaccination with 82% occurring in males whose median age was 21 years. The breakdown per age group of males is listed in Table 2 [8].

Table 2: Age groups and incidence of VAM among males following receipt of second dose of COVID-19 vaccine [8].
Choi et al reported a singular case of myocarditis induced sudden death after receipt of the Pfizer vaccination [9].
Tables 3 and 4 contain summaries of population studies and case series on COVID-19 VAM.
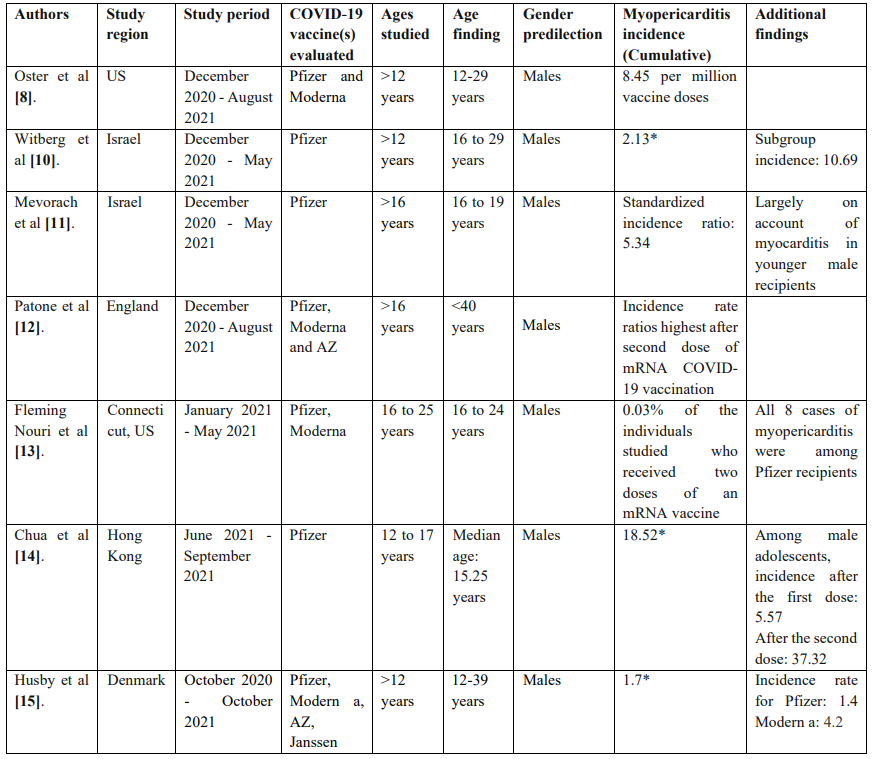
Table 3: Summary of population studies on COVID-19 VAM.
* Incidence rates are reported per 100,000 vaccinated individuals in the studied groups/subgroups.
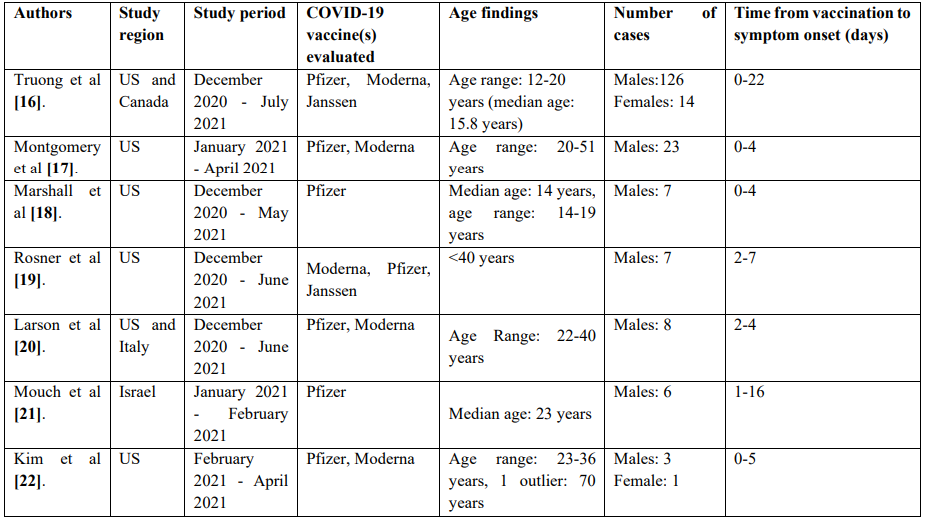
Table 4: Synopsis of non population studies (case series reports) of COVID-19 VAM.
As seen above, numerous studies have linked COVID-19 vaccines, especially mRNA vaccines including Pfizer and Moderna with myopericarditis. Despite these observations, the overall incidence of myopercaridits following COVID-19 vaccination including mRNA vaccination has been deemed low among all age groups and genders [23]. Further, myopericarditis has rarely been observed thus far following COVID-19 vaccination with adenoviral vector vaccines (Janssen and AZ). Majority of cases reported in the literature occurred among young males, within 30 days after vaccination, most often within 7 days of vaccine receipt.
Future possible areas of research include long term sequelae of COVID-19 VAM, its impact on future athletic pursuits, and the ability to seek COVID-19 vaccines in the future including booster doses. Lastly, the advent of childhood vaccination (for those <5 years of age) against COVID-19 poses many questions about vaccination related adverse events including COVID-19 VAM in this age group. Despite such speculation, and all the information quoted in the literature to date, protection conferred by COVID-19 vaccines against severe disease and death has shown significant promise, building the case for greater benefits than risks of vaccine receipt.
Acknowledgements:
We would like to thank S. Chadha, MD for sharing her wisdom and insight with us during the course of this paper.
References:
- Inciardi RM, Lupi L, Zaccone G, et al. (2020) Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 5(7): 819-824. [PubMed.]
- Fried JA, Ramasubbu K, Bhatt R, et al. (2020) The Variety of Cardiovascular Presentations of COVID-19.Circulation. 141(23): 1930-1936. [PubMed.]
- Daniels CJ, Rajpal S, Greenshields JT, et al. (2021) Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 6(9): 1078-1087. [Ref.]
- Gargano JW, Wallace M, Hadler SC, et al. (2021) Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 70: 977-982. [Ref.]
- Halsell JS, Riddle JR, Atwood JE, et al. (2003) Myopericarditis Following Smallpox Vaccination Among Vaccinia-Naive US Military Personnel. JAMA. 289(24): 3283-3289. [Ref.]
- FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. [Ref.]
- World Health Organization. (2022) [Ref.]
- Oster ME, Shay DK, Su JR, et al. (2022) Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December to August. JAMA. 327(4): 331-340. [Ref.]
- Choi S, Lee S, Seo JW, Kim MJ, Jeon YH, et al. (2021) Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J Korean Med Sci. 36(40): e286. [Ref.]
- Witberg G, Barda N, Hoss S, Richter I, Wiessman M, et al. (2021) Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 385(23): 2132-2139. [Ref.]
- Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, et al. (2021) Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 385(23): 2140-2149. [Ref.]
- Patone M, Mei XW, Handunnetthi L. et al. (2022) Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 28: 410-422. [Ref.]
- Fleming-Nouri A, Haimovich AD, Yang D, Schulz WL, Coppi A. et al. (2021) Myopericarditis in young adults presenting to the emergency department after receiving a second COVID-19 mRNA vaccine. Acad Emerg Med. 28: 802-805. [Ref.]
- Chua GT, Kwan MYW, Chui CSL, Smith RD, Cheung EC, et al. (2021) Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination. Clin Infect Dis. 28: ciab989. [PubMed.]
- Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, et al. (2021) SARS-CoV-2 vaccination and myocarditis or myopericarditis: population-based cohort study. BMJ. 375: e068665. [Ref.]
- Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, et al. (2022) Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults: Suspected Myocarditis After COVID-19 Vaccination. Circulation. 145(5): 345-356. [Ref.]
- Montgomery J, Ryan M, Engler R, et al. (2021) Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 6(10): 1202-1206. [Ref.]
- Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, et al. (2021) Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 148(3): e2021052478. [PubMed.]
- Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, et al. (2021) Myocarditis Temporally Associated with COVID-19 Vaccination. Circulation. 144(6): 502-505. [Ref.]
- Larson KF, Ammirati E, Adler ED, Cooper LT Jr, Hong KN, et al. (2021) Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation. 144(6): 506-508. [Ref.]
- Mouch SA, Roguin A, Hellou E, Ishai A, Shoshan U, et al. (2021) Myocarditis following COVID-19 mRNA vaccination. Vaccine. 39(29): 3790-3793. [PubMed.]
- Kim HW, Jenista ER, Wendell DC, et al. (2021) Patients with Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 6(10): 1196-1201. [Ref.]
- Heymans S, Cooper LT. (2022) Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 19: 75-77. [Ref.]