>Corresponding Authors : Wakgari Oljira Fayisa
>Article Type : Review Article
>Volume : 2 | Issue : 2
>Received Date : 17 June, 2022
>Accepted Date : 12 July, 2022
>Published Date : 15 July, 2022
>DOI : https://doi.org/10.54289/JCVR2200108
>Citation : Ayana GM, Fayisa WO. (2022) Study on Prevalence of Equine Strongylosis and Associated Risk Factors in and Around Guder, Oromia, Ethiopia. J Clin Vet Res 2(2): doi https://doi.org/10.54289/JCVR2200108
>Copyright : © 2022 Ayana GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Review Article | Open Access | Full Text
1Department of Veterinary Science, College of Agriculture and Veterinary Sciences, Ambo University
2Jima Rare District Office of Agriculture, Horo Guduru Wollega Zone, Western Oromia, Ethiopia
*Corresponding author: Wakgari Oljira Fayisa, Jima Rare District Office of Agriculture, Horo Guduru Wollega Zone, Western Oromia, Ethiopia
Abstract
A Cross sectional study was conducted from April to June 2019 in and around Guder town to estimate the prevalence of strongyle infection and assess associated risk factors in the study area in donkeys, mules and horses. A total of 384 animals were randomly selected from different peasant associations in the study area and examined during the study period. Fecal examination for the detection of strongyle eggs was performed using floatation technique. The overall prevalence of strongyle parasites was 25.26% (97 from 384) with 14.58% in donkeys, 6.77% in mules and 3.9% in horses. The study has also showed variation in prevalence of strongyle parasites among different body condition scores and among purpose, higher prevalence was recorded in poor body condition (16.4%) and draft (14.06 %). The risk factors; body condition and purpose status of the equines in the study area were significantly associated with the occurrence of strongyle parasites (p<0.05). In conclusion the current study revealed that stronglosis was found to be the major problem in the study area; hence Strategic deworming and minimizing overworking and extensive open grazing should be implemented to reduce pasture contamination.
Keywords: Equine; Guder; Prevalence; Risk Factors; Strongyle; Strongylosis
Abbreviations: S. Vulgaris: Strongylus Vulgaris, BCS: Body Condition Scores
Introduction
The equine population of the world is 122.4 million (40 million donkeys, 15 million mules’ 43.3 million Horses and [1]. In the distribution pattern, 98% of all donkeys, 97% of all mules and 384% of all horses are found in the developing countries. The number of equines in Africa is in the range of 17.6 million comprising 11.6 million donkeys, 2.3 million mules3.7 million horses [2].
Equines (donkeys, mules and horses) play an important role as working animals in many parts of the world, employed for packing, riding, carting and plough. Equine power is vital for both rural and urban transport system which is cheap and provides the best alternatives in places where the road network is insufficiently developed [3].
Equines as a means of transport for men and materials provide livelihood to a number of rural and semi urban population of the world. They have a prominent position in agricultural systems of many developing countries. It is suggested that donkey can play a great role in the frame works of food security and social equity of high food in secure countries. In areas away from roads, many people use mule’s donkey as well as horses to transport food and other supplies to villages [4,5].
Ethiopia is one of the developing countries in Africa, which is predominantly an agricultural country with over 85% of its population engaged in agricultural activity [6,7]. The country has the highest equine population probably with the highest density per square kilometre in the world [3] and it has a total of 6.9% and 42.4% in the world and Africa equine respectively [8].
Ethiopia possess about 5.02 million donkeys, 2.75 million horses and 0.63 million mules [9]. Equine play an important role in the transportation of products, fodder, fuel, wood, agricultural inputs and construction and waits materials [10]. Equine endoparasites may be divided into three categories: nematodes, or roundworms; cestodes, or tapeworms; trematodes, or flukes. Parasites are assigned to these categories according to their morphology, or structure. Growth and life cycles of parasites within each group are generally distinct from those of the other groups. The roundworms are by far the most economically important internal parasites of equines. Internal parasites continue to be a significant threat to the health of equines. Even under proper management equines will become infested with internal parasites. Internal parasites of equines are of veterinary importance in many countries, where current methods of control rely almost entirely on the use of antihleminthes [11].
Infections caused by strongyles constitute a severe impediment to successful equine management due to debility and death of animals, particularly when heavy burdens are involved. Even light infections can affect the development and the performance of equines. The adult worms produce lesions in the gut wall as they feed and larvae make destructive migrations in various tissues of the animal body Strongylus vulgaris (S. vulgaris) stands out as being particularly dangerous because the larvae develop in the mesenteric arterial system causing arthritis and thrombosis with serious consequences [12]. Patterns of transmission vary greatly with climate and management, therefore no worming program is universally applied [11]. Strongylosis is the most common and economically devastating disease of equine clinically infected equine exhibit signs of unthrift ness, anemia, colic and diarrhoea [13].
Investigation about this parasite were concentrated mainly on few areas such as [9] and [14] around Bahirdar were reported that with a prevalence of 100% and 83.85% overall, [15] and [10] and [16] in which they reported, 100%, 100%, 100% and 98.2%, 87.7% in donkeys of, Wonchi, highland, Wollo province, Dugda Bora and western high land of Oromia, Gondar respectively. Although different investigation has been done in different part of Ethiopia, there is no data on Equine strongylosis in and Guder Town, Toke Kutaye district, west Shewa Zone, Oromia, Ethiopia. Therefore, this study was conducted to determine the prevalence of the disease, identify responsible Strongyle species, and assess potential risk factors in and around Guder town.
Materials and methods Study Area
The study was conducted in Guder town, western Shewa zone of Oromia regional state which located at 137 km west of the capital city. The area is found at the altitude range from 1250-3200 m a.m. The climatic condition of an annual rainfall and temperature ranging from 800-1100mm and 16-22% respectively. The rainfall is bimodal with a short rainy season from February to May and long rainy season from June to September. The total animal population in Guder town is 145,4384 cattle, 50,413 sheep, and goat, 24,772 poultry; 4,7384 horses, mule, and donkey. According to the district report, the total human population in Guder town is 134,767. Out of this 66,492 is male and 68,275 is female.
Figure 1: Map of study area (source: created by Arch Map 10.2 software).
The Study Animal
A total of 384 equine species were studied which consists of horse, donkey, and mule in the toke kutaye woreda. Each animal was considered for study after knowing history, species, body condition, altitude of the area and approximate age. Age of animal was determined by using age determination chart develop based on dentition. Equine species were grouped in to two groups (categories). The animal under two-year age young and beyond two years (above two years) classified as adult [17].
Study Design
A cross-sectional study design was used to estimate the prevalence of strongyle infection in horses, mules and donkeys in the study area. All horses, mules and donkeys presented to the clinic with suggestive of clinical manifestation of strongyle infection were considered.
Sample Size Determination
A random sampling technique used to recruit an animal for the study. Because there was no information about the prevalence of the strongylosis disease in the study area. Sample size was calculated according to [18]. The expected prevalence 50% with 95% confidence interval and 5% desired absolute precision (d=0.05).
n = Z2.p (1-p)/ d2
Where: n = sample size, p = expected prevalence, d = desired level precision, 1.96= the value of “Z” at 95% level of confidence, Pexp = 50% and d = 5% so n=384.
Materials and Chemicals
The materials and chemicals used in this study include: two beakers, microscope, slide, cover slip, disposal glove, pippet, pistle and mortal, bottles, chemicals (formalin 10%) and tea strainer. The flotation solution used in this study was salt solution.
Method of Data Collection
Fecal samples were collected directly from the rectum of 384 suspected animals (donkeys, mules and horses) present to Guder veterinary clinic from Guder town and the surrounding using disposable glove and put in air and watertight sample vials. The collected samples were properly labelled with the necessary information and soon transported to veterinary parasitology laboratory. Samples were processed and examined on the day of collection and samples not processed on collection day were preserved in formalin for the next day to be processed. The samples were processed by simple test tube floatation technique and diagnosis was based on the observation of strongyle eggs in microscopic examination of fecal samples using 10x and sometimes 40x magnification power.
Data Analysis
After collection of all the necessary data, it was coded on prearranged coding sheet. Data was entered into excel 2010 and analyzed through SPSS version 20 software. Tables were present results of pertinent findings. Association of host risk factors with strongyle parasite positives was calculated. A statistically significant association between variables is considered to exist if the compute p-value is less than 0.05.
Results
Out of 384 equines examined 97 (25.25%) were found to be positive for strongyles. The equine was confirmed to be positive depending on age, sex, body condition and species seen from sample processed and examined. The overall prevalence was found to be 25.25% and the species-specific prevalence was 14.56%, 6.77% and 3.9% in donkeys, horses and mules, respectively.
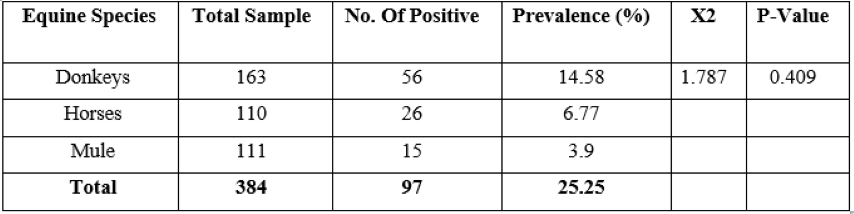
Table 1: Prevalence of strongylosis among donkey, horse and mule
There was no statistically significant difference (p >0.05) on the prevalence of the strongyle’s infection in different species of equines in the study area (Table 1).
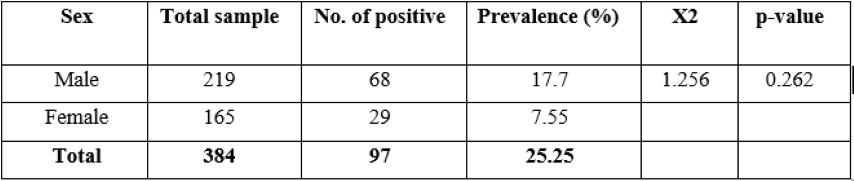
Table 2: Prevalence of strongyles infection by sex of equines species.
Sex based prevalence was 17.7% and 7.5% in male and female equine species, respectively. There was no statistically significance difference (p >0.05) in the prevalence of strongyles infection in different sex of equine species in the study area (Table 2).
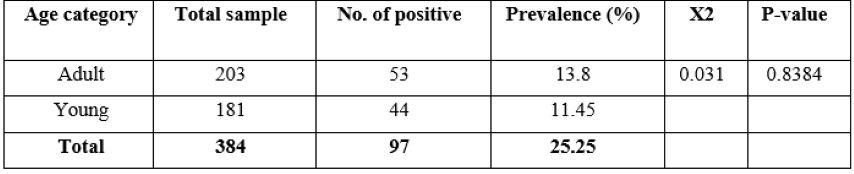
Table 3: Prevalence of strongyles infection young and adult equines.
The study animals were categorized into two age groups, young (<2 years) and adult (>2 years of age). The prevalence on age bases was 13.8% and 11.45% in adult and young equines, respectively. There was no statistically significant variation (p >0.05) in the occurrence of strongyles infection with the two age groups (Table 3).
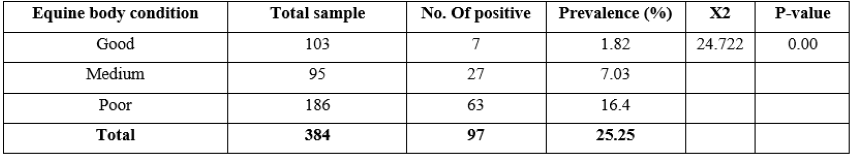
Table 4: Prevalence of strongyles infection in different body condition scored equines.
Out of 103 equines with good body condition score, 7 (1.82%) were positive for strongyles infection, whereas from 95 equines with medium body condition score, 27 (7.03%) were positive for strongyles infection and from 186 equines with poor body condition score, 63 (16.4%) were positive for strongyles infection. There was statistically significant difference (P <0.05) in the prevalence of strongyles infection with regard to the body condition score (Table 4).
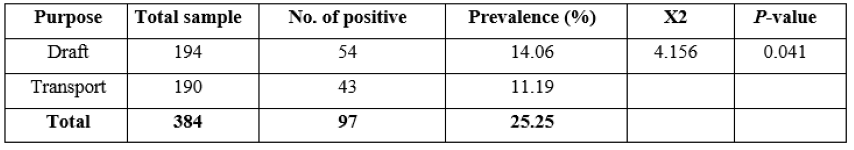
Table 5: Prevalence in different purpose of equines
Prevalence in different purpose of equines
Out of 384 equines used for purpose of draft score, 149 (38.8%) were positive for strongyles infection, whereas from 384 equines used for purpose of transport score, 43(11.2%) were positive for strongyles infection. There was statistically significant difference (P <0.05) in the prevalence of strongyles infection with regard to the purpose score (Table 5).
Discussions
The prevalence of GI equine stronglosis among equine species in the current study in Toke kutaye district revealed that from total sample 384 examined 97 equine species were positive which counts 25.25% prevalence. The current study also revealed that there is lower prevalence of stronglosis when compared with the study reported by [19]. They reported that 93% in Bereh, 87% in Boset and 95% in Ada’a respectively. The present study is also less than to the work reported by [12] and [3] from East Shewa and Adaa, Akaki and Boset of East Shewa that revealed 100% and 99% prevalence respectively. This difference might be due to the difference in environmental conditions, sample size, sampling time, management practice favoring the survival of the larvae of the parasite and availability of antihelementics in the study area.
Out of 384 fecal samples examined 14.56%, Donkeys, 3.9% mules and 6.77% horses were found to be positive for GI equine strongylosis. There was no significant variation (p>0.05) among equine species. This insignificance agrees with that of [2]. The prevalence of the current study was also lower than as compared with the results of, [6,10,20] and [18] in which they reported, 100%, 100%, 100% and 98.2%, 87.7% in donkeys of Wonchi, highland of Wollo province, Dugda Bora and western high land of Oromia, Gonder respectively. This variation might be due to difference in agro-ecology and density of equine population in the area and the practice of use of antihelementics therapy for equines in the study area.
The present study shows that prevalence of equine strongylosis was not sex influences. The prevalence was indicated that male equine species 17.7% and female equines species 7.52% were observed. This study revealed that there was no statically variation between the two sex groups of equine species (p>0.05). This finding is in agreement with the findings of [10] and [19]; both reported there was no statistically significant difference in between sex. The present finding is not in agreement with the work done by [15] and [5]. They reported a significance difference P< 0.05 between males and females, with higher prevalence rate reported in females. This could be associated with the more workload in males than females, which could create most of males get less chance for grazing the pasture but mostly get non pasture feeds when compared to females and, females usually have not more cares because females are mostly used as breeding purpose.
There was a significance variation (p<0.05) association between body condition scores (BCS) of in equine strongylosis infection. The study revealed association between body condition scores and the level of GIT equine strongylosis infection in equine species in Toke kutaye district. This implies that body condition scores were used to indicate the burden of parasites in good, medium and poor was 1.82%, 7.03% and 16.4% respectively. This study shows that prevalence of equine strongylosis higher in poor body condition and secondly highly occurred in medium body condition compared with good body condition. In the current study which is in agreement with the study of [2] and [19]. Likewise, equine with poor body condition have higher chance of harboring the parasites. This could be due to the fact that animals with poor body condition might be immunocompromised probably due to malnourishment and higher workload and as a result be exposed to stongylosis.
The prevalence of equine strongylosis on the fecal examination in adult equine species was 13.8% and in young equine species 11.45%. There is no statistically significant variation between the age of the equine (p>0.05). This agrees with the work of [21] in two age groups because of care to management system which avoid communal grazing of animals of all age groups together. The present finding is not agreement with [22] who reported there is higher prevalence in young animals when compared to adult and old equines. This could be due to the difference in management, feeding and practice of deworming difference among the age groups of equines in the study areas because young equines in the study area feed both grazing and gets supplementary feeds when the mothers goes to market and for other packing activities.
There was statistically significance difference (P < 0.05) among equines used for different purposes. Concerning the purposes for which the animals were kept. Equine that were used for draft purpose was found to be with higher prevalence of stongylosis than animals used for transport, and this might be confounded by the difference in the management given to these groups of animals. There was no a habit of giving especial care for the equines used for draft-purpose such as deworming and supplementary feed rather than the chance of extensive grazing for these animals on work, as the result getting chance of infection as compare to use for the purpose of draft and transport in the study area. But equines which were used for the purpose of draft and transport were habit of giving especial care such as deworming and supplementary feed so that reduce the chance of getting infection.
However, there is no previous data reported regarding this issue at all.
Conclusion and Recommendations
In Ethiopia there is a less modern transportation system in rural areas and some towns. Equines are the most useful means of transport in both industrial and farm products in rural and towns. Helminthes parasites were playing a great damage in equine species and have high economic negative feedback. Due to this detailed study conducted the veterinarian render their performance in control, management system health care and housing system of equine species. Based on this study a lot of equines graze in free pasture causes contamination of equine strongylosis and facilitate prevalence of the disease. Sex, age, body condition and the purpose of equine have a great effect in prevalence of equine GI strongylosis. In the present study, body condition and the species of equine showed highly significant variation for the occurrence of strongylosis. Based on the above conclusion the following recommendations were for warded.
● Free gazing of a lot of equine species in small grazing area should be avoided.
● Strategic GI equine strongylosis control should be identified.
● Decrease high workload in equine species to keep its body weight in normal state
References
- Abayneh T, Fiseha G and Gizaw T. (2002) The potential role of donkeys in lantillage in central Ethiopia. Bulletin of Animal Heal h and Production in Africa. 50: 172-178. [Ref.]
- Chapman and Klein R. (2002) Gastrointestinal helmintthes of ponies in Louisiana: comparison of species currently prevalent with those presen20 years ago. Journal of Parasitology. 88(6):1130-1134. [PubMed.]
- Getachew M, Feseha G, Trafford S and Reid J. (2008) A survey of seasonal patterns in strongyle fecal worm egg counts of working equines of the central midlands and lowlands. Tropical Animal Health and Production. 40: 637-642. [Ref.]
- Yoseph F, Mengistu F, Teklu T, Firwe Y and Betere D. (2008) Seasonal variation in the parasite burden and body condition of working donkeys in east Shewa and West Shewa Regions of Ethiopia. Tropical Animal Health and Production. 37: 35-45. [Ref.]
- Yoseph F, Smith A, Mengistu F, Teklu T, Firwe Y, et al. (2005) Seasonal variation in the parasite burden and body condition of working donkeys in east Shewa and West Shewa Regions of Ethiopia. Tropical Animal Health and Production. 37(1): 35-45. [Ref.]
- Food and agriculture organization. (1999) Production year book Food and agriculture organization of United Nations. [Ref.]
- Alemayehu L. (2004) Case study on reproductive activity of equines in relation to environment factors in central Ethiopia, Berlin: hum bold university of Berlin, PhD thesis, Autopsy end in Poland. Veterinary Parasitology. 58: 99-108. [Ref.]
- Feseha G. (1997) Disease and health problem of equines. The professional handbook and equines (accompanied by E.D, saves, densen). 3rded, Whiter books. 202-226. [Ref.]
- Ethiopia Research Organization. (1999) National animal health research programme strategy document, Addis Ababa, Ethiopia. [Ref.]
- Duncan JL and Pirie HM. (1985) The pathogenesis of single experimental infections with Strongylus vulgaris in foals. Research in Vet. Sci. 18: 82-93. [Ref.]
- Belay. (2006) Preliminary study on helmenthosis of equines in south and north Wollo zones. Veterinary Parasitology. 140: 289-295. [Ref.]
- Ogbourne CP. (1975) Epidemiological studies on horses infected with nematodes of the family Trichonematidae. Into J parasitology. 5: 667-720. [Ref.]
- Urquhart G, Amour J, Duncan A, Dunn F and Jenin W. (1996) Veterinary Parasitology, 2nded, Black Well Science Ltd. London UK. 212-219. [Ref.]
- Getachew M, Feseha G, Trafford S and Reid J. (2008) A survey of seasonal patterns in strongyle fecal worm egg counts of working equines of the central midlands and lowlands, Tropical Animal Health and Production. 40: 637-642. [Ref.]
- Jemal, S. (2008) The study on the prevalence of GI strongyles among equine species in and around Assela and to assess the magnitude of this parasitism in relation to age sex and management system DVM thesis Faculty of Veterinary Medicine Addis Ababa University Debrezeit, Ethiopia. [Ref.]
- Feseha G, Alemu K, Freed I, Abele Y and Ketema A. (1999) Donkey Utilization and management in Ethiopia. African, Caribbean, European Union (ACP-EU). Technical Centre for Agricultural and Rural Cooperation (CTA), Wageningen. 46-52. [Ref.]
- Tolla M, Ketema T and Firaol T. (2013) Prevalence of Gastrointestinal Parasites of Horses and Donkeys in and around Gondar Town Open Journal of Veterinary Medicine. 3: 267-272. [Ref.]
- Svendsen. (1997) Donkey abroad the professional handbook of the donkey (3rd) edition. [Ref.]
- Thrusfield M. (2007) Veterinary epidemiology. 3 ed. Singapore, Blackwell Science. 233. [Ref.]
- Ayele G and Dinka A. (2010) Study on strongyle and parascaris parasites population working donkeys of central Shoa, Ethiopia Faculty of Veterinary Medicine, Addis Ababa University. Liveock Research for Rural Development. 22(12). [Ref.]
- Alemayehu R and Etaferahu Y. (2013) Gastrointestinal Parasites of Equine in South Wollo Zone, North-eastern Ethiopia. Global Veterinary. 11(6): 824-830. [Ref.]
- Soulsby E J L. (1968) Helminthes, Arthropods and Protozoa of Domesticated Animals (7thedn) Bailliere Tindal London. 167-174. [Ref.]