>Corresponding Author : Sevidzem Silas Lendzele
>Article Type : Research Article
>Volume : 2 | Issue : 1
>Received Date : 25 April, 2022
>Accepted Date : 05 May, 2022
>Published Date : 08 May, 2022
>DOI : https://doi.org/10.54289/JCVR2200104
>Citation : Kumar P. (2022) Sustainable Livestock Systems and Concurrent Challenges: A Mini Review. J Clin Vet Res 2(1): doi https://doi.org/10.54289/JCVR2200104Lendzele SS, Koumba AA, Rodrigue MN, Mavoungou JF. (2022) Foot and Mouth Disease in Cameroon: A Systematic Review to Support its Progressive Control. J Clin Vet Res 2(1): doi https://doi.org/10.54289/JCVR2200104
>Copyright : © 2022 Lendzele SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access | Full Text
1Organisation for Milk and Meat Production (PLEB), Adamawa, Cameroon
2Department of Biology and Animal Ecology, Institute of Research in Tropical Ecology, Libreville, Gabon
3University of Science and Technique Masuku, Franceville, Gabon
4International University of Libreville, Gabon
*Corresponding author: Sevidzem Silas Lendzele, Organisation for Milk and Meat Production (PLEB), Adamawa, Cameroon, Department of Biology and Animal Ecology, Institute of Research in Tropical Ecology, Libreville, Gabon
Abstract
Foot-and-mouth disease (FMD) is an infectious viral transboundary disease of even-toed animals, caused by the foot-and-mouth disease virus (FMDV). The objective of this paper is to provide reviewed data on the epidemiology, risks, control and the existing gaps on FMD in Cameroon from 1990 to 2021 to support its progressive control. Relevant and available documents (n = 45) on FMD in Cameroon such as peer-reviewed papers (n = 27), dissertations (n = 11), conferences (n = 5) and technical reports (n = 2) were used. Studies on the molecular and serological epidemiology, the role of carrier/mobile animals, the evaluation of immune-cross protection between serotypes and the quantification of serotype-specific transmission parameters using mathematical models have been discussed. The environmental drivers of FMD in cattle markets, abattoir and herd-level, as well as risk factors such as husbandry practices, communal grazing, regional/international livestock trading system, transhumance and fomites, are presented. As international borders remain porous with poor FMD monitoring infrastructure at veterinary checkpoints in a country with no candidate vaccine, vaccination program and commercial vaccines available, farmers are left with no option other than to manage the disease with traditional formulations and veterinary pharmaceuticals mostly antibiotics. Tri-Solfen® (TS), a wound dressing formulation was efficient in managing FMD in Cameroon. The current gaps in FMD knowledge in Cameroon are presented. There is a need for a comprehensive epidemiological study in the major cattle rearing regions of Cameroon. A regional study to identify all the risk factors for the transmission of FMD is required. A socio-economic impact study of FMD is required in Cameroon.
Keywords: Foot-and-Mouth Disease; Serotypes; Epidemiology; Risk Factors; Transmission; Control; Gaps Analysis
Abbreviations: FMD: Foot-And-Mouth Disease, FMDV: Foot-and-Mouth Disease Virus, TS: Tri-Solfen, SAT: South African Territories, CAR: Central African Republic, PCP: Progressive Control Pathway, Eufmd: European Commission for Foot-And-Mouth Disease Control, GFRA: Global Foot-And-Mouth Disease Research Alliance, PACE: Pan African Control of Epizootics, IAH: Institute for Animal Health, OIE: Organisation for Animal Health, BVI: Botswana Vaccine Institute, AMR: Antimicrobial Resistance
Introduction
Foot-and-mouth disease is a highly contagious viral disease of even-toed animals belonging to the family Picornaviridae and genus Aphthovirus. It is among the diseases that greatly affect the progress of the livestock industry in SSA because its endemicity does not only reduces animal production and health but also hinders international trade with FMD free regions [1]. This disease is caused by seven known serotypes (O, A, C, Asia 1, South African Territories (SAT) 1, SAT 2, SAT 3) with characteristic ecologically distinct evolutionary lineages or subtypes within serotypes and limited cross-immunity between serotypes or lineages [2]. Cameroon is found in FMD pool 5 with four serological types (O, A, SAT 1, SAT 2) endemic to countries in the Central and West African subregions. In Cameroon, molecular studies have confirmed that serotypes-O, A, and SAT 2 are endemic [3-5]. In 2019, SAT1 topotype X was reported for the first time in bovine epithelial tissues from North Cameroon [6,7]. Indeed, serological epidemiological studies have established the occurrence of five serotypes-O, A, SAT1, SAT 2, SAT 3 [8,9]. As international borders remain permeable without strict veterinary checkpoints to identify, isolate and prevent sick animals from entering the country, the risk of incursions of FMD viruses is very obvious as shown in studies conducted at border levels between Cameroon and neighbouring countries such as Chad, Central African Republic (CAR), and Nigeria [7,10-14].
The risk-based control approach using the global progressive control pathway for FMD control (PCP-FMD) is the target for Cameroon as it is currently in the PCP-FMD stage one [7]. FMD transmission risk sources such as communal grazing, mobile livestock especially cattle, the role of carrier animals, role of fomites (a fomite is any material or object that can harbour an infectious disease and probably contaminate the environment and hosts), husbandry system, high livestock density, and the environment around herds, abattoirs and cattle markets have been examined and identified to be potential transmission areas to target for its mitigation [14-18].
The control of FMD in Cameroon is a major concern as no vaccination programs are ongoing and its management is mostly conducted by farmers themselves using veterinary pharmaceuticals mostly purchased from black markets and only a few of them buy from recognised pharmacies [17]. The field trial of the trivalent FMD vaccine (O, A, SAT 2) reduced clinical FMD but did not stop persistent infections [5]. The field trial of TS, a topical formulation improved the overall demeanour of animals, hastened recovery time, wound healing time and quick return to pasture compared to animals treated with antibiotics and non-treated counterparts [18]. The management of the disease with antibiotics could have public health consequences such as the risk of the development of antibiotic resistance [19]. Therefore, vaccination or the use of an alternative such as TS could be suggested for FMD control in Cameroon.
The present study aims at conducting a systematic review of relevant documents available from 1990 to 2021 on FMD epidemiology, risk-based studies, control options and gap analysis to support its progressive control.
Materials and Methods
Study area: Cameroon is a bilingual country and is located at the northeastern end of the Gulf of Guinea between the equator and the tropics. Cameroon is in the form of a triangle with a 700 km base and a 1200 km side with a total surface area of 475000 Km². It is found between longitude 9° to 16° east and latitude 2° to 13° north. It forms part of Central African countries, where it shares a boundary to the South with Equatorial Guinea, Gabon and Congo, to the West with Nigeria, to the East with the Central African Republic and Chad. Cameroon is a major cattle rearing region and supplier of livestock products to neighbouring countries in the Central African subregion. The major livestock rearing regions of Cameroon are the Far North, Adamawa, North and North West. The livestock population in Cameroon includes 31 million poultry, 6 million cattle, 7 million small ruminants, one million pigs, 150,000 donkeys and 15,000 horses [14]. The distribution of cattle throughout the national territory is as such: 37.5% in the Far North, 33.9% in Adamawa, 11.6% in the North, 8% in the North West, 6.3% in the East and 2.7% in the West[14]. The present study focused on reviewing available data on FMD in all the regions of Cameroon.
Literature search methods and selection criteria: A web-based systematic search to get information from the published research literature (peer-reviewed papers and dissertations), the consultation of technical reports of the LANAVET-MINEPIA and conference presentations made at the European Commission for Foot-and-mouth disease Control (EuFMD) and Global Foot-and-mouth disease Research Alliance (GFRA) meetings as well as the EuFMD/FAO/OIE reports on FMD in Cameroon. The published documents were gotten via Google, Google Scholar, ResearchGate, and websites of local and international institutions working on FMD in Cameroon. Only those documents reporting on the epidemiology, transmission risk, and control measures of FMD from January 1990 to August 2021 were selected for this study. Based on the stated inclusion criteria, a total of 45 relevant and complete documents were retained for this systematic review, excluding documents that had no information on the study country and objectives (Fig. 1).
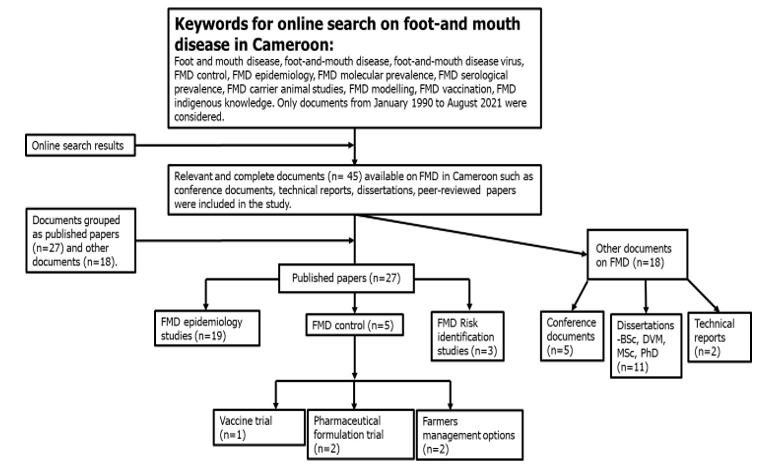
Fig. 1: Workflow on the collection of relevant Scientific documents on FMD in Cameroon
Results And Discussion
Epidemiological findings on FMD
Foot-and-mouth disease is endemic in Cameroon where the health and productivity of about 7 million cattle, 3.5 million sheep, 4 million goats and 1.7 million pigs is threatened by it [14]. Studies on FMD in Cameroon between 1931 and 1988 showed the circulation of serotypes O and A [20]. The national veterinary laboratory (LANAVET) in Garoua isolated serotypes A, O, SAT 2, SAT 3 from cattle and pig samples between 1987 and 1998. Subsequently, studies in the Adamawa region from 2000 to 2006, highlighted the presence of serotypes SAT 2, O, and A [4]. In 2006 within the framework of the Pan African Control of Epizootics (PACE)-Cameroon program, samples collected, part analysed in LANAVET, revealed the circulation of serotypes SAT 2, O, A, and were confirmed by the Institute for Animal Health (IAH)-Pirbright. According to the Cameroon report (Prepared by the LANAVET) from 2005 to 2011 to the International Organisation for Animal Health (OIE), it showed that in 2005 four serotypes (A, O, SAT 1, SAT 2) were detected, but samples from 2007 to 2011 were not typed. In 2011, samples were collected from FMD outbreak areas of Adamawa and North regions by the LANAVET staff and sent to Botswana Vaccine Institute (BVI) for virus isolation, phylogenetic analysis and vaccine matching. From the analysis, SAT 2 was isolated from samples collected from the North and Adamawa. In 2015, three serotypes (SAT 2, O, A) were identified in Ngaoundere [5]. In general, four FMD serotypes (A, O, SAT 1, SAT2) have been confirmed to be circulating in Cameroon (Fig. 2). The serotypes identified in Cameroon have also been identified in neighbouring Chad [21]. The SAT1 topotype X was reported for the first time in Cameroonian (samples from the North region) and Nigerian cattle (Fig. 2) [6,7]. The occurrence of this SAT 1 topotype X between the two countries is not surprising as they face the challenge of uncontrolled animal movement across permeable international borders that allows the contact of animals, thus facilitating the incursion of FMD viruses and spread. Similarly, the SAT 2 topotype VII identified in Chadian livestock was similar to that reported in 2015 in Cameroon [21], confirming that uncontrolled cattle movement in endemic SSA is a central mechanism for FMD introduction and distribution [22-24].
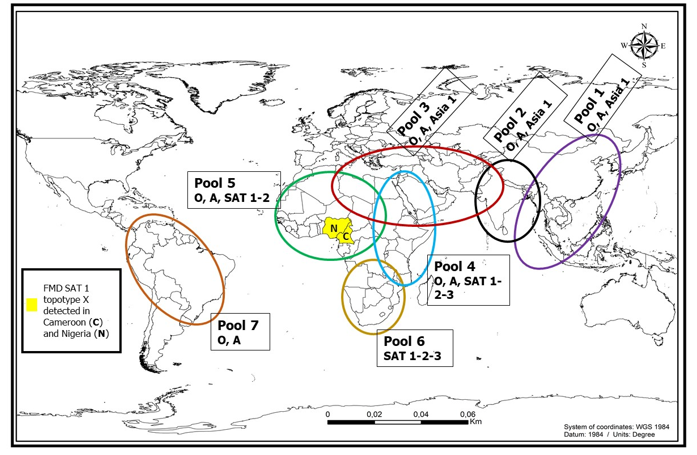
Fig. 2: World distribution of FMD serotype pools indicating the recently identified SAT 1 topotype X identified in Cameroon and Nigeria. This modified map does not indicate the FMD sporadic, endemic and free areas of the world
The role of small ruminants in FMD epidemiology is currently being considered in Cameroon. Studies conducted in the North and Adamawa regions found that subclinical small ruminants (goats and sheep) were infected with FMD and most seriously when they are reared with other animal species as well as graze around wildlife areas [14]. In Cameroon, the mixed-species herd husbandry practice is very common with cattle kept alongside other ruminant species like goats, sheep and rarely pigs [14]. The possibility of cross-transmission of FMD between animal species through contact has already been reported in Kenya [25]. Although the control of FMD by only vaccinating large ruminants without including small ruminants worked in certain settings, this has led to the negligence of the role of small ruminants in FMD epidemiological studies. However, the role of small ruminants in sustaining FMD transmission chains in an endemic setting is still debated.
Recent advances have been made in the epidemiological studies of FMD in Cameroon by the OSU and the University of Edinburgh (UoE) researchers through the use of. mathematical modelling to project FMD transmission in the Far-North and Adamawa. Serological data from an FMD endemic setting (Far-North region of Cameroon) was used to probe strain-specific transmission immuno-dynamics [26]. They discovered that for serotypes SAT 2, SAT 3, O, a model assuming lifelong immunity fitted better for serotypes SAT 1 and O and this better fit model suggested that immunity could wane over time. They noticed that serotype O had the highest force of infection (FOI) and longest duration of immunity, SAT1 and O displayed endemic dynamics, type A displayed epidemic dynamism and SAT 2, SAT 3 did not sustain local chains of transmission. Similarly, simulating the transmission of FMD among mobile herds in the Far-North region indicated that grazing areas observed in the field (≤ 5km radius) resulted in multiple epidemic peaks in a year [27]. A study conducted on subclinical and mobile animals indicated the potential transmission risk by such groups [11]. In Adamawa, the role of carrier animals in FMD spread was examined and a redefinition of the carrier animal status for endemic settings was suggested [15]. Herdsmen report on FMD has also been an important tool to track infected cases, but how accurate this might be has been verified in studies conducted in the Adamawa region. A study conducted to validate herdsmen sensitivity and specificity in Adamawa concluded that although farmers could identify the disease with high sensitivity, this should be conducted in parallel with serological testing [28].
From published technical documents and articles, the highest number of serotypes was recorded in the Far-North region (O, A, SAT1, 2, 3) [8] (Table 1). The North, Adamawa and North-West regions have the same number of serotypes (O, A, SAT 1, SAT 2) [3,4,9,12;14]. The other regions such as Littoral and Center have low serotype diversity, while for the South-West and South regions there is no information on the circulating serotypes (Table 1).
Table 1: FMD serotypes within the ten regions of Cameroon from 1990 to 2021.
| Region | Serotypes identified | Reference |
|---|---|---|
| Adamawa | 0, A, SAT 1, SAT 2 | [3, 4, 5, 9, 14, 20] |
| Center | SAT 2 | [14] |
| East | SAT 2, O | [14] |
| Far Nortd | O, A, SAT 1, SAT 2, SAT 3 | [5,8,13,26] |
| Nortd | O, A, SAT1, SAT 2 | [6,7] |
| Nortd West | O, A, SAT 1, SAT 2 | [14] |
| West | O, SAT 1, SAT 3 | [14] |
| Soutd West | Not typed* | [14] |
| Soutd | Not typed* | [14] |
*Samples from sites in the South and South-West regions were positive but were not serotyped.
According to table 2, FMD serotypes O and A were the most frequent serotypes in Cameroon as they were detected in almost all the years that samples were serotyped. From the nationwide FMD confirmed cases reported from 1990 to 2021, it could be deduced that the disease is widespread and endemic in Cameroon (Table 2).
Risk-based identification findings: The transmission life cycle of FMD has been studied and remains complex for endemic settings. Some aspects of the foot-and-mouth disease virus (FMDV) life cycle such as its persistence in the environment, the occurrence of subclinical animals (domestic and wild) and the possibility of transmission from small ruminants to cattle remain subjects for debate. The already identified potential risk sources for FMD transmission in the indigenous cattle population of Cameroon are cattle density, livestock trading practice, livestock markets/abattoir environments, husbandry systems, uncontrolled livestock movements, porous international borders and fomites. Some of these transmission sources have been fully described [29].
Table 1: FMD confirmed serotypes in animal species from 1990 to 2021.
| Reported year | Animal species | Circulating serotypes | Reference |
| 1931 to 1959 | Cattle | O | [20] |
| 1975 | Cattle | A | [20] |
| 1976 | Cattle | A | [20] |
| 1980 | Cattle | O | [20] |
| 1981 to 1984 | Cattle, Pigs | O | [20] |
| 1985 to 1987 | Cattle, Pigs, Goats, sheep | A, SAT3 | [20] |
| 1988 | Cattle, Pigs | O, A, SAT2, SAT3 | [20] |
| 1989 | Cattle | O, A | [14] |
| 1996 | Cattle | O, A,SAT2 | [14] |
| 1998 | Cattle | SAT2 | [14] |
| 2004 | Cattle | O, A | [3] |
| 2005 | Cattle | O,A,SAT1, SAT2 | [14] |
| 2006 | Cattle | O,A,SAT1, SAT2, SAT3 | [4, 14] |
| 2014 | Cattle | O,A,SAT1,SAT2,SAT3 | [8] |
| 2015 | Cattle | O,A,SAT1,SAT2,SAT3 | [14, 26] |
| 2018 | Cattle | O,A,SAT2 | [5, 12] |
| 2019 | Cattle, Sheep, Goats | O, A, SAT1,SAT2 | [6, 9, 14] |
| 2020 | Cattle | SAT1 | [7] |
Livestock trading system: Livestock trade in Cameroon involves transactions in cattle markets between cattle owners who pay taxes to Government officials upon entry. It is reported that 32% of herders declare buying their cattle from conventional cattle markets [14]. Trade animals are usually accompanied by other animals of the same herd (a herd is the smallest homogenously mixing unit of animals[8]). The unsold animals, plus those accompanying are returned to the herd. Livestock places such as cattle markets have been identified as risk areas for the transmission of infectious diseases [30]. Such risk could occur if unsold animals and those that accompanied them to the market return with FMD viruses to contaminate the other animals in the herd.
The major cattle production zones of Cameroon are found in the North-West, Adamawa, Far-North and North regions. The animals bred in such major production areas are sold in other regions with major markets found in the cities of Yaounde, Douala, Bafoussam, Limbe, Ebolowa and Bertoua. International export markets for local production include Nigeria, Gabon, Congo and Equatorial Guinea. Another type of trading system is that which involves transit livestock from the Central African Republic, Chad, Sudan and Ethiopia that pass through Cameroon to Nigeria, Gabon, Congo and Equatorial Guinea (Fig. 3). Interestingly, 70% of Cameroonian herdsmen can easily identify FMD in animals in a cattle market but 8% would still buy an animal suffering from FMD because of their low prices. A survey in the Far-North region reported that about 10% of the cattle sold by pastoralists were reportedly sick at the time of the sale [31]. In Kenya, the purchase of sick animals from stock markets by small-scale dairy farmers have been reported as risk factor for FMD dissemination [32]. From a national survey conducted in 2012, it was found that 31% of those who would buy an infected animal are from the North-West followed by the North (14%) and East (12%) [14]. It is clear that despite having sound knowledge of the disease, farmers still sell FMD affected animals and this ugly practice promotes the spread of the disease.
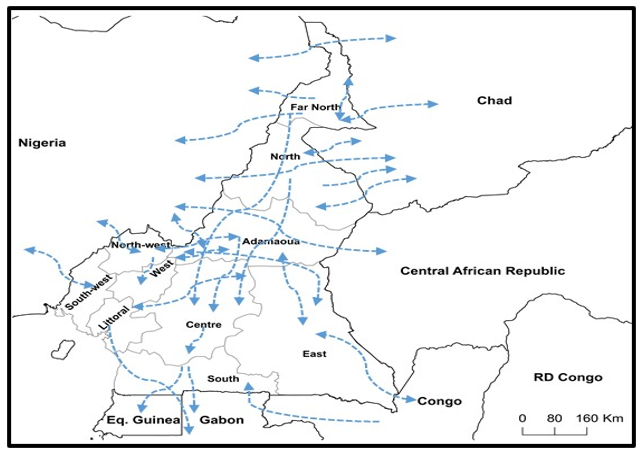
Fig. 3: Livestock trade network at regional and international levels
Husbandry practices that promote FMD spread: Past surveys indicated that 92% of Cameroonian herders reported cases of FMD, but during outbreaks, only 30% separated sick animals from the rest of the herd, the remaining 70% kept sick animals with the rest of the herd [14]. A recent report on farmers management practices during FMD outbreak periods to curb the spread of the disease in Adamawa showed that the practice of herd isolation was very difficult based on the free livestock grazing husbandry practice [17]. It was noticed in the majority of the smallholder dairy farms in Adamawa that most herders used the hand-milking technique without disinfecting their hands as well as used the same utensils between animals that could promote contamination in their herds [14].
Despite great knowledge on FMD by Cameroonian herdsmen, the majority of them still conduct local husbandry practices that facilitate within-herd and between herds spread. Some of these practices are an exchange of bulls for reproduction purposes, allowing sick animals to mix with non-sick counterparts, transhumance towards FMD wild reservoir sites with buffaloes, antelopes, warthogs, and mixed livestock farming (whereby herders mix herds of different susceptible animal species such as goats, sheep, but rarely pigs with their cattle). Studies conducted in the North and Adamawa reported serotype O in small ruminants (sheep and goats) [9]. Additionally, the study in the North identified that herders who practised grazing of small ruminants with other animal species reported high FMD contamination. Mixed livestock grazing practice leads to high animal contact and has been reported as a risk factor for FMD spread in west, central, east and southern Africa [32, 33, 34] and Asia [35]. It has been highlighted that dearth of information on good management practices by herders could be attributed to the disorganized nature of the herders, where only a few of them belong to farmer organizations.
Transhumance: Transhumance is another major virus transmission mechanism and involves the southward movement of animals into game reserves, the Guinean savannah, the northwestern grasslands and the forest agro-ecological zones. These zones represent habitats for buffaloes, antelopes and warthogs. It is known that buffaloes are natural reservoir hosts for SAT 1, SAT 2, SAT 3 FMD viruses [36]. Moreover, FMDV has already been recovered from subclinical buffaloes in South East Asia [37]. It has already been reported that transhumance herds usually come in contact with other herds at pasture, drinking and places where they camp [11, 38]. In practice, transhumant animals often come in contact with other animals during the process and upon return and this could lead to contamination with new FMD viruses that are brought back to sedentary animals that did not go on transhumance. Additionally, some instances of transboundary trade herds camping overnight with native herds lead to direct contact[26]. Thus, transhumant animal contact could be a major transmission risk source for FMD in Cameroon.
The occurrence of wild buffalo niches in SSA permits the maintenance of FMD transmission. In some West African countries (Benin, Burkina Faso and Nigeria) and Central Africa countries (Cameroon, Central African Republic (CAR), Chad, Democratic Republic of Congo and Gabon) buffalo subspecies have been identified. For Cameroon, Syncerus caffer brachyceros and Syncerus caffer nanus occur [39]. Buffalo samples from Gabon and Nigeria all tested negative for FMD, but no buffalo samples were available from Cameroon for testing in this study [39]. However, buffalo samples from neighbouring countries such as DR Congo, Chad and CAR were positive. Further, when wildlife species were screened in neighbouring Nigeria, FMD was detected [40]. It is well known that the Cape buffalo (Syncerus caffer caffer) is a long term maintenance host for SATs [36]. Hence, cross-border transhumance where livestock share grazing, drinking and camping points with wildlife and buffaloes, exposes them to FMD infection.
The porosity of international borders: One of the FMD incursion sources is likely through transboundary trade animals from the neighbouring Central African Republic and Chad and during transit to Nigeria, Gabon and Equatorial Guinea. The importation of live animals and animal products through seaports and the international airports from endemic countries into Cameroon without thorough inspection cannot be rolled-out. Every year, transhumant animals come to the East and Adamawa, from Chad and the Central African Republic. Similarly, every year, several heads of ruminant livestock come on transhumance from neighbouring Chad, Niger and Nigeria to the Far-north Region of Cameroon thereby increasing animal density and contacts that permit FMD introduction and distribution. Further, these animals entering the country are generally not diagnosed or thoroughly inspected for FMD at the different veterinary checkpoints before allowing them to enter the country. To avoid the introduction and spread of FMD viruses in the national territory, a competent epidemiological surveillance infrastructure is required at different livestock entry points of the country.
Abattoirs and livestock markets: The modern abattoirs located in some Cameroonian cities such as Yaounde, Douala and Ngaoundere belong to the state-owned livestock production and exploitation corporation (SODEPA). However, there are several slaughter slabs with low veterinary service inspection. Information from available sources indicates that the abattoirs and livestock markets in Cameroon have no diagnostic facilities and limited technical capacity to identify some diseases like FMD. However, the veterinary service inspection is based on visual examination. Although the thorough inspection is supposed to be conducted in abattoir and livestock markets, it is rather unfortunate that such services do not exist for FMD as veterinary chief of centres in some major cattle production areas do not have data on FMD cases. Abattoirs have been identified as risk areas for the spread of infectious diseases because it receives animals from everywhere and some of them have been reported to be sick [10]. The handling of animal carcasses requires high biosecurity measures to avoid personal and environmental contamination. If infected animal carcasses, secretions and excretions get into the environment, it could lead to the spread of highly contagious diseases such as FMD in such zones. Samples collected in abattoirs and cattle markets in six locations within the main agro-ecological zones of Cameroon revealed high contamination in Douala and Bertoua. Genetic analysis of one of the samples led to the isolation of serotype O of the East African-3 lineage.
Regarding the livestock trading system of Cameroon, it is characterized by trade animals from neighbouring Chad passing through the Far-North Region of Cameroon to the Borno state in Nigeria. During transit in Cameroon, they mix with resident herds during transhumance towards the Waza park. There, they come in contact with wild reservoirs through the sharing of pasture, drinking and camping areas.
The animals coming in through the North and Adamawa regions from the Central African Republic could be contaminated upon contact with those found in the Benoue and Bouba-Njidda game reserves on their way to the Adamawa state of Nigeria. Besides transit to Nigeria, there is transit to Gabon and Equatorial Guinea across all intervening regions to the South region. Major cattle markets of Cameroon receives animals from different regions and neighbouring countries with some of the animals subclinically and clinically infected. The animals are transported to markets on foot and by lorries. Animals from different herds are often packed in the same lorry. Similarly, during trekking to livestock markets, two or more animal herds usually come in contact either on their way to the market or upon return. During market days a subset of cattle merchants buy animals and they are stocked in one large pen before their transportation from one local market to the next. This kind of market system promotes the spread of infectious diseases especially FMD between localities. It should be noted that not all animals taken to these markets are sold and if they come in contact with sick counterparts and are carried home, they could contaminate other susceptible ones.
Fomites: The implication of fomites in FMD spread is another avenue of research that is neglected globally. The factors suspected to be responsible for FMD spread include various domestic/wild animals, birds, men, truck tires and postal materials. Persistence of the FMDV on surfaces is a potential transmission risk and include animal farms (49 weeks), soil (1 to 21 weeks), manure (1 to 24 weeks), vegetables (1 week), H2O (3-14 weeks), house flies (10 weeks), ticks and haematin of ticks (15-20 weeks), wool of sheep (2 weeks), hair of cattle (4-6 weeks), and cattle skin used as cloth [41]. For FMDV to persist for longer periods on these surfaces, optimal environmental conditions must occur. Weather variables such as temperature, relative humidity and pH have been reported to affect FMDV persistence in endemic settings [42].
In 2016 the first author of this paper was interested in knowing the role of stable flies especially the blood-sucking Stomoxys niger (Fig. 4a) in the contaminative transmission of FMDV during outbreaks. Preliminary findings of the study conducted during the 2016 FMD outbreak in Ngaoundere of the Adamawa region of Cameroon indicated that this fly vector’s mouthparts (Fig. 4b) and legs (Fig. 4c) were contaminated with the FMDV (Fig. 4d) RNA and legs frequently contaminated than mouthparts but mouthparts had a high viral load [41]. The association of the biting predilection sites of different stable flies with areas on the host model where FMD clinical lesions are produced indicated a strong association with these two variables. This shows that flies coming in contact with open sores containing FMDV could lead to their contamination and if they land around open sores of susceptible host this could lead to successful inoculation of the virus [43].
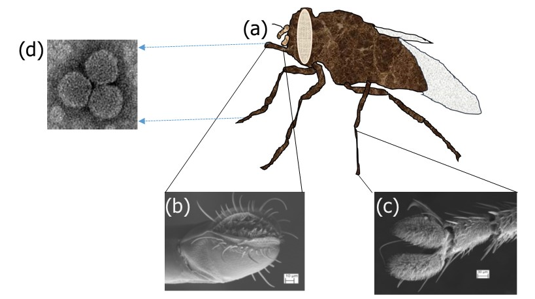
Fig. (4a-d): Stomoxys and its body parts involved in the contaminative transmission of FMDV. (a)Adult Stomoxys; (b) Scanning electron microscopy of proboscis; (c) Scanning electron microscopy of tarsus; (d) FMD virus (millions of FMDV particles (size 0.03μm) presumably attach to the hairs of the pulvillus of legs when the fly lands on infected body parts. They could also attach to the peristomal teeth and labellae of probosci when the fly tries to imbibe infected blood, excretions and secretions
Control efforts: The average cost of FMD treatment in Cameroon is estimated at 80, 000 FCFA/herder/year on drugs and the average annual expenditure on drugs for its treatment is estimated at 32,000,000,000 FCFA (32 billion FCFA). There are no vaccination programs and available commercial vaccines to curb the spread of FMD in Cameroon. However, vaccine trials, topical formulation trials and studies to know the different FMD management options by herdsmen have been conducted.
Vaccination: The MINEPIA together with LANAVET in 2013, acquired 150 000 doses of an FMD trivalent vaccine prepared by the BVI using endemic serotypes (A, O, SAT2). The first FMD vaccination campaign in Cameroon was launched in March 2014. This pilot campaign targeted dairy herds in four divisions of two regions-Adamawa (Vina and Mbere divisions) and North-West (Mezam and Bui divisions). The major pitfall of this campaign is that there was no Post Vaccination Monitoring (PVM) record available to say if the vaccine was efficient or not. These weaknesses and challenges in the implementation of FMD vaccination in Africa have already been reported [44,45].
Tri-Solfen: The Australian wound dressing formulation, TS (Medical Ethics Pty Ltd, Australia) is registered for use in cattle and small ruminant husbandry in Australia and New Zealand and has recently been registered for FMD therapy in Lao People’s Democratic Republic (PDR). This formulation offers numerous advantages over current therapies. TS has been confirmed as providing significant pain relief and more rapid healing of wounds and lesions [46]. Further, with a pH of ~2.7, it potentially has a viricidal impact, avoiding the need for other treatments, including antibiotics. The dressing contains two local anaesthetics (lignocaine and bupivacaine), adrenalin and an antiseptic (cetrimide) in a gel formulation that creates a barrier effect, numbing the pain of lesions, rapidly reducing their infectivity, and hastening to heal, potentially reducing the weight loss in affected individuals.
From the results of the first clinical trial of TS in Cameroon, farmer observations indicated that the TS treatment group had a more rapid return to eating with cessation of excessive salivation, and more rapid return of mobility (walking) with the absence of overt lameness compared to the commonly used antibiotic Moore-Oxy® (MO) (oxytetracycline HCL 5%, Hebei Kexing Pharmaceutical Co. Ltd., Shijiazhuang City, China) and the non-treated control group [18]. 100% of farmers expressed their desire that the product is made available for use in their region and cost-effective modelling indicated that TS therapy only costs 2.5 USD, imposes no additional financial burden on farmers, with the treatment likely to be provided at a similar or reduced cost to current treatment choices. Further, TS can have a significant impact on improving lesion treatment and reducing viral transmission during outbreaks, whilst addressing risks of antimicrobial food safety and antimicrobial resistance (AMR) issues. Farmers own control methods
Chemotherapy: As no FMD vaccination programs, commercial vaccines and TS are available to farmers in Cameroon, they have devised other strategies to manage the disease. A field study in major cattle markets where most drug dealers come to sell their products was surveyed and it was noticed that farmers purchased antibiotics and antiparasitics to manage FMD. Only a few of them practised herd hygiene (cleaning of wounds) and herd isolation. Procaine (antibiotic) was the most frequently used management option (51.23%) followed by procaine plus medicinal plants (13.22%) and medicinal plants (10.74%) [17]. The most frequently prescribed drugs for the management of FMD were antibiotics followed by antiparasitic and lastly by anti-inflammatories. The majority of the drug sellers prescribed antibiotics precisely MO whose common name was boldly written on its bottle as “Likki Bauru” in Fulfude, meaning FMD drug [17].
Husbandry management practice: Another herd management practice that facilitates FMD transmission in herds is the use of sick cases to speedherd transmission so that they can treat them at once to reduce management costs. In the endemic Far-North region of Cameroon with high FMD serotype diversity, the issue of how much cross-protection between FMD serotypes might prevent transmission was investigated for serotypes O and A. From the simulation runs, it was concluded that cross-immunity would not prevent FMD infections in the Cameroonian context [13].
Medicinal plants: Despite the frequent use of different veterinary pharmaceuticals to manage FMD in Cameroon, traditional medicinal plants are also widely used. The treatment of animals with these medicinal plants is often associated with antibiotics [17]. In the Far North region of Cameroon 12 medicinal plants are commonly used by farmers to manage FMD [47]. Interestingly, two of the medicinal plants known as Bosica senegalensis and Tapinanthus dodoneifolius possesses active compounds that are responsible for several biological activities, reason why they are used by farmers to manage FMD. However, only 10% of farmers interviewed in the different cattle markets of Adamawa confirmed the use of only medicinal plants to treat FMD. Although these medicinal plants are widely available, farmers still prefer antibiotics like ‘procaine’ that is cheap and available than traditional preparations that are difficult to prepare, time-consuming and more expensive.
FMD progressive control pathway for Cameroon: The PCP-FMD is a stepwise framework that assists countries to progress towards achieving freedom from FMD (with or without vaccination) after official approval by the OIE (Fig. 5). Cameroon is presently in stage one [7] of the PCP-FMD and a risk-based control plan is ongoing. The Cameroonian Government is willing to control FMD and this is manifested through the training of staff of Divisional delegations. The Government also offers financial support to LANAVET to boost its testing capacity. To improve its diagnostic infrastructure to satisfy the increasing demands, LANAVET is currently creating branches in the different regions of the country. There are no records of FMD regional control initiatives in Central Africa, but the Government’s commitment to other regional control initiatives against epizootics is evidence of its readiness in case of an eventual regional control initiative.
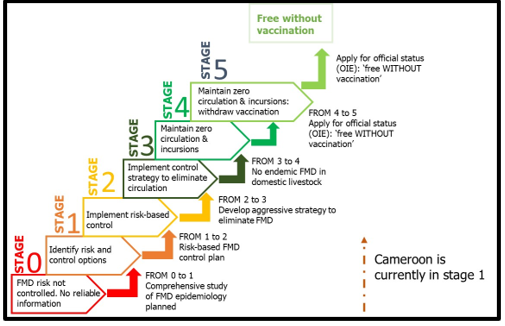
Fig. 5: The different PCP stages for FMD control with Cameroon in stage 1. Source: Modified from https://www.fao.org/ag/AGAinfo/commissions/docs/PCP/PCP_en.pdf. Abbreviations: FMD, foot-and-mouth disease; OIE, World Organisation for Animal Health; PCP, Progressive Control Pathway
Gaps analysis
Epidemiological surveillance studies: Apart from the comprehensive national study on FMD carried out in 2012, no systematic nationwide data on this disease is available. There is a need to increase the FMD testing capacity, to conduct regular field surveys at regional and border levels to generate robust epidemiological data.
Socio-economic impact studies: There is a need to update the socio-economic impact of FMD on the different livestock breeder categories.
Risk-based studies: There is a need to study the role of wild animals in FMD dissemination as studies on this subject has not yet been realised in Cameroon. The role of small ruminants in FMD transmission in mixed herds with cattle should also be studied. The role of environmental contaminants such as soils, air at an abattoir, cattle markets, and herds as well as other fomites (swabs from lorries transporting cattle from one cattle market to the next, livestock owners and health workers) should be conducted to design biosecurity measures typical of the Cameroonian setting, required to curb the spread of FMD. As preliminary studies have already indicated that stable flies could serve as mechanical vectors, there is a need to collect samples during an outbreak and non-outbreak periods to determine virus persistence and to conduct further experiments to define their vectorial capacity.
FMD control studies: The FMD control phase in Cameroon will require that robust, credible and updated national epidemiological data is available to guide tailored control. For this, researchers, state veterinarians and those in the private sector could contribute to generating such information. The lessons learned from the last vaccine campaign could help inform future vaccine trial efforts in Cameroon. The first field trial of TS was very successful and this product is already authorised for use in Cameroon (authorisation number: MA no DRL0101120/CMR).
Conclusion
Four FMD serological types (SAT 1, SAT 2, O, A) circulates in the animal populations of Cameroon. Abattoir and cattle markets environments represent transmission risk areas. Stomoxys niger could be involved in FMD spread. Questionnaire studies revealed the possibility of animal contact during transhumance and the difficulty faced by farmers to quarantine their animals during outbreaks to curb the spread of FMD. No vaccination programs and commercial vaccines are available in Cameroon. The first tri-solfen clinical trial was very successful and was authorised for use in Cameroon. The main gaps in FMD research are lack of regular surveillance data, no comprehensive socio-economic FMD impact studies, studies on the role of wild animal hosts in its transmission is not available.
References
- James AD, Rushton J. (2002) The economics of foot-and-mouth disease. Rev. Sci. Tech. 21: 637-644. [Ref.]
- Knowles NJ, Samuel AR. (2003) Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 91: 65-80. [Ref.]
- Bronsvoort MC, Nfon C, Hamman SM, et al. (2004) Risk factors for herdsman-reported foot-and-mouth disease in the Adamawa Province of Cameroon. Prevent. Vet. Med. 66: 127-139. [Ref.]
- Bronsvoort BM, Anderson J, Corteyn A, et al. (2006) Geographical and Age-Stratified distribution of Foot and Mouth disease virus-seropositive and probang-positive cattle herds in the Adamawa province of Cameroon. Vet. Rec. 158: 299-308. [Ref.]
- Bertram MR, Delgado A, Pauszek SJ, et al. (2018) Effect of vaccination on cattle sub-clinically infected with foot-and-mouth disease virus in Cameroon. Prevent. Vet. Med. A. 155: 1-10. [Ref.]
- Bertram MR, Dickmu S, Palinski RM, et al. (2019) Genome sequences of four foot-and-mouth disease virus SAT 1 topotype X isolates from Cameroon. Microbiol. Resour. Announce. 8: e01243-19. [Ref.]
- Ehizibolo DO, Fish IH, Brito B, et al. (2020) Characterization of transboundary foot-and-mouth disease viruses in Nigeria and Cameroon during (2016). Transbound. Emerg. Dis. 00: 1-14. [Ref.]
- Ludi A, Ahmed Z, Pomeroy LW, et al. (2016) Serotype Diversity of Foot-and-Mouth-Disease Virus in Livestock without History of Vaccination in the Far North Region of Cameroon. Transbound. Emerg. Dis. 63: e27-e38. [Ref.]
- Sevidzem SL, Mamoudou A, Mavoungou JF, et al. (2019) Serological Epidemiology of Foot-and-Mouth Disease among Sedentary Mixed-species Herds in Adamawa Region, Cameroon. J. Adv. Microbiol. 17: 2456-7116. [Ref.]
- Mebanga AS, Beloko SL, Mamoudou A. (2020) Prevalence and risk factors of foot-and-mouth disease in the cattle market of Garoua-Boulai and in the transhumance zone of Lome and Djerem in East Cameroon. Int. J. Biol. Chem. Sci. 14: 2799-2806. [Ref.]
- Schnell PM, Shao Y, Pomeroy LW, et al. (2019) Modeling the role of carrier and mobile herds on foot-and-mouth disease virus endemicity in the Far North Region of Cameroon. Epidemics. 29: 100355. [Ref.]
- Bertram MR, Bravo de Rueda C, Garabed R, et al. (2018) Molecular epidemiology of foot-and-mouth disease virus in the context of transboundary animal movement in the far North Region of Cameroon. Front. Vet. Sci. 5: 320. [Ref.]
- Xiao N, Cai S, Moritz M, Garabed R, Pomeroy LW. (2015) Spatial and temporal characteristics of pastoral mobility in the far north region, Cameroon: data analysis and modeling. PLoS One. 10: 131-139. [PubMed.]
- Lendzele SS, Armel KA, Rodrigue MN, et al. (2021) Clinical Foot-and-Mouth Disease in Non-Vaccinated Smallholder Dairy Cattle in Adamawa Region, Cameroon: Prevalence, Farmer’s Knowledge and Practices. J. Vet. Med. Surg. 5: 4-40. [Ref.]
- Bronsvoort BM, Handel IG, Nfon CK, et al. (2016) Redefining the “carrier” state for foot-and-mouth disease from the dynamics of virus persistence in endemically affected cattle populations. Sci. Rep. 6: 29059. [Ref.]
- Motta P, Thibaud P, Saidou MH, et al. (2018) Cattle transhumance and agropastoral nomadic herding practices in Central Cameroon. BMC Vet. Res. 14: 214. [Ref.]
- Sevidzem SL, Mavoungou JF, Mintsa NR. (2019) Veterinary pharmaceuticals sold in cattle markets for the management of foot-and-mouth disease and flies in Vina division (Adamawa-Cameroon). Dairy Vet. Sci. J. 10: 2. [Ref.]
- Lendzele SS, Mavoungou JF, Kong AB, et al. (2020) Efficacy and application of a novel topical anaesthetic wound formulation for treating cattle with Foot-and-Mouth disease: a field trial in Cameroon. Transbound. Emerg. Dis. 00: 1-12. [Ref.]
- Ngom RRBV, Foyet HS, Garabed R, et al. (2020) Human Health Risks Related to Penicillin G. and Oxytetracycline Residues Intake Through Beef Consumption and Consumer Knowledge About Drug Residues in Maroua, Far North of Cameroon. Front. Vet. Sci. 7: 478. [Ref.]
- Ekue N, Tanya V, Ndi C. (1990) Foot-and-mouth disease in Cameroon. Trop. Anim. Health Prod. 22: 34-36. [Ref.]
- Abdel‐Aziz AI, Aurore R, Relmy A, et al. (2019) Seroprevalence and molecular characterization of footand‐mouth disease virus in Chad. Vet. Med. Sci. 00: 1-8. [Ref.]
- Ularamu HG, Ibu JO, Abenga JN, et al. (2020) Incursion of Foot-and-Mouth Disease (FMD) Serotype O East Africa Topotype -3 (O/EA-3) in Nigeria. Nig. Vet. J. 41. [Ref.]
- Kerfua SD, Shirima G, Kusiluka L, et al. (2018) Spatial and temporal distribution of foot-and-mouth disease in four districts situated along the Uganda-Tanzania border: Implications for cross-border efforts in disease control’. Onderstepoort J. Vet. Res. 85: a1528. [PubMed.]
- Jori F, Etter E. (2016) Transmission of foot and mouth disease at the wildlife/livestock interface of the Kruger National Park, South Africa: Can the risk be mitigated? Prevent. Vet. Med. 126: 19-29. [Ref.]
- Chepkwony EC, Gitao GC, Muchemi GM, et al. (2021) Epidemiological study on foot-and-mouth disease in small ruminants: Sero-prevalence and risk factor assessment in Kenya. PLoS One. 16: e0234286. [Ref.]
- Pomeroy LW, Bjørnstad ON, Kim H, et al. (2015) Serotype-Specific Transmission and Waning Immunity of Endemic Foot-and-Mouth Disease Virus in Cameroon. PLoS One. 10: e0136642. [Ref.]
- Kim H, Xiao N, Moritz M, et al. (2016) Simulating the Transmission of Foot-And-Mouth Disease Among Mobile Herds in the Far North Region, Cameroon. J. Artif. Soc. Soc. Simul. 19. [Ref.]
- Morgan KL, Handel IG, Tanya VN, et al. (2014) Accuracy of herdsmen reporting versus serologic testing for estimating foot-and-mouth disease prevalence. Emerg. Infect. Dis. 20: 2048-2054. [Ref.]
- Paton DJ, Gubbins S, King DP. (2018) Understanding the transmission of foot-and-mouth disease virus at different scales. Curr. Opinion Virol. 28: 85-91. [Ref.]
- Motta P, Porphyre T, Handel I, et al. (2017) Implications of the cattle trade network in Cameroon for regional disease prevention and control. Sci. Rep. 7: 43932. [PubMed.]
- Profitós JMH, Moritz M, Garabed RB. (2013) What to do with chronically sick animals? Pastoralists’ management strategies in the far north region of Cameroon. Pastoralism. 3: 8. [Ref.]
- Nyaguthii DM, Armson B, Kitala PM, et al. (2019) Knowledge and risk factors for foot-and-mouth disease among small-scale dairy farmers in an endemic setting. Vet. Res. 50: 33. [Ref.]
- Sinkala Y, Simuunza M, Pfeiffer DU, et al. (2014) Challenges and Economic Implications in the Control of Foot and Mouth Disease in Sub-Saharan Africa: Lessons from the Zambian Experience. Vet. Med. Int. 12. [Ref.]
- Ekwem D, Morrison TA, Reeve R, et al. (2021) Livestock movement informs the risk of disease spread in traditional production systems in East Africa. Sci. Rep. 11: 16375. [Ref.]
- Sansamur C, Arjkumpa O, Charoenpanyanet A, Punyapornwithaya V. (2020) Determination of Risk Factors Associated with Foot and Mouth Disease Outbreaks in Dairy Farms in Chiang Mai Province, Northern Thailand. Animals. 10: 512. [Ref.]
- Wekesa SN, Sangula AK, Belsham GJ, et al. (2015) Characterisation of recent foot-and-mouth disease viruses from African buffalo (Syncerus caffer) and cattle in Kenya is consistent with independent virus populations. BMC Vet Res. 11: 17. [Ref.]
- Buckle K, Bueno R, McFadden A, et al. (2021) Detection of Foot-and-Mouth Disease Virus in the Absence of Clinical Disease in Cattle and Buffalo in South East Asia. Front. Vet. Sci. 8: 691308. [Ref.]
- Bronsvoort M, Tanya VN, Kitching RP. (2003) Foot and mouth disease and livestock husbandry practices in the Adamawa Province of Cameroon. Trop. Anim. Health. Prod. 35: 491-507. [Ref.]
- Di Nardo A, Libeau G, Chardonnet B. (2015) Serological profile of foot-and-mouth disease in wildlife populations of West and Central Africa with special reference to Syncerus caffer subspecies. Vet. Res. 46: 77. [Ref.]
- Atuman YJ, Kudi CA, Abdu PA, et al. (2020) Seroprevalence of Foot and Mouth Disease Virus Infection in Some Wildlife and Cattle in Bauchi State, Nigeria. Vet. Med. Int. 8. [Ref.]
- Sevidzem SL, Mamoudou A, Dickmu S. (2019) Risk Factors for the Contamination of Wild Stomoxys niger niger Macquart 1851 (Diptera: Muscidae) with the Foot-and-Mouth Disease Virus. Curr. Res. Agric. Sci. 6: 95-108. [Ref.]
- Mielke SR, Garabed R. (2019) Environmental Persistence of Foot and Mouth Disease Virus Applied to Endemic Regions. Transbound. Emerg. Dis. 1-12. [Ref.]
- Sevidzem SL, Mavoungou JF, Zinga-Koumba RC, M’batchi B. (2020) Alighting Dipterous Insects on Cattle are Associated to Contaminative Transmission of Foot-and-Mouth Disease During Epidemics in Ngaoundere Cameroon. Entomol. Faunist - Faunist. Entomol. 73: 132-144. [Ref.]
- Maree FF, Kasanga CJ, Scott KA. (2020) Challenges and prospects for the control of foot-and-mouth disease: an African perspective. Vet. Med.: Res. Rep. 202: 168-194. [Ref.]
- Jackson B, Harvey Y, Perez-Martin E. (2021) The selection of naturally stable candidate foot-and-mouth disease virus vaccine strains for East Africa. Vaccine. 39: 5015-5024. [Ref.]
- Windsor PA, Earp F, MacPhillamy I, et al. (2020) A new topical therapy for Foot-and-mouth disease addresses animal welfare and other issues. Vet Med: Res Rep. 11: 99-107. [Ref.]
- Vougat RRBN, Foyet HS, Garabed RB, Ziebe R. (2015) Antioxidant activity and phytochemical constituent of two plants used to manage foot-and-mouth disease in the Far North Region of Cameroon. J. Intercult. Ethnopharmacol. 4: 40-46. [Ref.]