>Corresponding Author : Ozlem Barut Selver
>Article Type : Mini Review Article
>Volume : 2 | Issue : 1
>Received Date : 26 May, 2022
>Accepted Date : 07 June, 2022
>Published Date : 10 June, 2022
>DOI : https://doi.org/10.54289/JORVC2200106
>Citation : Ciftci MD and Selver OB. (2022) Clinical Evaluation of Corneal Neovascularization: A Brief Review. J Ophthalmic Res Vis Care 2(2): doi https://doi.org/10.54289/JORVC2200106
>Copyright : © 2022 Ciftci MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mini Review Article | Open Access | Full Text
Assoc. Prof. Ege University, Department of Ophthalmology, Izmir, Turkey
*Corresponding author: Ozlem Barut Selver MD, Assoc. Prof. Dept. Ophthalmology, Ege University Medical Faculty Hospital 35100 Bornova-Izmir, Turkey
Abstract
The healthy cornea is optically transparent tissue. Basically 3 features of the cornea provide its transparency: avascularity, constant water content and regular arrangement of collagen fibers. When the cornea loses its optically clear structure, it inevitably causes a decrease in visual acuity. Infection, inflammation, limbal stem cell deficiency, tumors and contact lens usage can cause corneal neovascularization. Disruption of the balance between angiogenic and antiangiogenic factors on the ocular surface plays a role in the pathogenesis of corneal vascularization. Most of the treatment options used in daily practice are effective when neovascular vessels are immature.
Keywords: Cornea; Neovascularization; Angiogenesis; Ocular Surface; Avascularity
Abbreviation: Cornv: Corneal Neovascularization, VEGF: Vascular Endothelial Growth Factor, PGF: Placenta Growth Factor, BfGF: Basic Fibroblast Growth Factor, MMP: Matrix Metalloproteinases, ECM: Extracellular Matrix, PDGF: Platelet Derived Growth Factor, PEDF: Pigment Epithelium Derived Factor, sVEGFR: Soluble Vascular Endothelial Growth Factor Receptor, KID: Keratitis-Ichthyosis-Deafness, NSAID: Non-Steroidal Anti-inflammatory Drugs, PDT: Photodynamic Therapy
Introduction
The cornea is the most important refractive element of the eye. Angiogenesis is formation new blood vessels from pre-existing vascularity [1]. Corneal neovascularization (CorNV) is formation of new vascular structures in previously avascular cornea. There are many pathological situations that cause CorNV. Etiological factors can be broadly thought of as initiating at least one of two common pathways: inflammation and limbal stem cell deficiency [2]. Disruption of the balance between proangiogenic and antiangiogenic factors towards to proangiogenic factors is involved in the pathogenesis of corneal vascularization [3]. In this review, we aim to summarize briefly pathophysiology of CorNV, corneal disorders associated with neovascularization and treatment options for these disorders.
Corneal Avascularity and Pathophysiology of Neovascularization
The cornea has no lymphatic or blood vessels. It provides its oxygen and nutritional needs from tear film and aqueous humor [4,5].
Avascular structure of the cornea is defined as angiogenic privilege [6]. There is a homeostasis in the cornea where proangiogenic and antiangiogenic factors are in balance.
Upregulation of proangiogenic factors accompanied by the downregulation of antiangiogenic factors promotes new blood vessel formation [7]. Neovascularization process starts via activation of vascular endothelial cells. Activated endothelial cells release proteolytic enzymes which degrade basement membrane and extracellular matrix (ECM). After degradation of ECM, endothelial cells migrate and proliferate through previously avascular cornea [2]. Sprouting and intussusception are two different branching mechanisms that used for CorNV. Proliferation and migration of endothelial cells to angiogenic stimulus is called as sprouting whereas enlargement of existing capillary plexus without cell proliferation is called as intussusception [8,9].
Newly formed blood vessels are leaky and unstable. In this stage, if proangiogenic stimuli cease to predominate nascent vessels can regress. After pericytes and smooth muscle cells surround the new vessels, they become stable and mature [2].
Epidemiology and Etiologic Factors of the Corneal Neovascularization
Neovascular diseases of the cornea and other parts of the eye represent a public health problem. Lee P et al [10] found that 4% of the US population has CorNV. A 14-year retrospective study from Italy CorNV was diagnosed 10.4% of patients that referred to the San Raffaele Cornea Unit [11].
A wide range of inflammatory, infectious and degenerative diseases may induce CorNV. Table 1 summarizes diseases associated with corneal neovascularization.
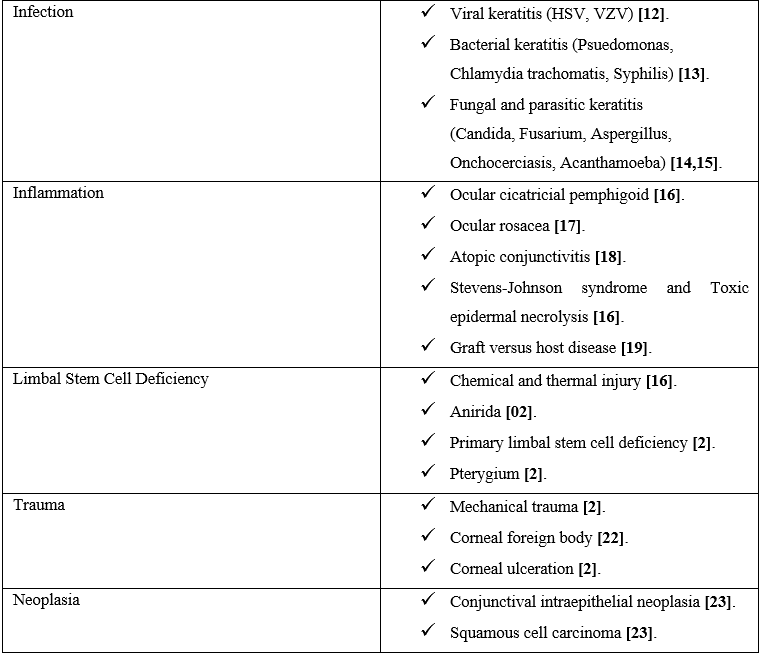
Table 1:Disorders associated with corneal neovascularization.
Proangiogenic and Antiangiogenic Factors
Corneal avascularity is a result of balance between proangiogenic and antiangiogenic factors [3].
A. Proangiogenic Factors
Vascular endothelial growth factor (VEGF) has important role in age-related macular degeneration and retinal vascular diseases. It is also upregulated in vascularized corneas in human and animal studies [24,25]. There are 5 members of
VEGF family. These are VEGF-A, -B, -C, and -D and placenta growth factor (PGF). They bind tyrosine kinase cell-surface VEGF receptors. The most important VEGF member that causes neovascularization is VEGF-A in both retina and cornea [26]. VEGF is secreted by many cell types like macrophages, T cells, retinal pigment epithelial cells, astrocytes, and smooth muscle cells. It promotes several steps of angiogenesis such as vascular leakage, liberation of endothelial cells from vessels, their migration and proliferation [27]. VEGF secretion is promoted by both inflammation and hypoxia [28].
Another proangiogenic factor is basic fibroblast growth factor (bFGF). It causes endothelial cell activation and also induces the activation of matrix metalloproteinases (MMP) via tyrosine kinase receptors [29].
Matrix metalloproteinases are a group of zinc-binding proteolytic enzymes. They participate in extracellular matrix (ECM) remodeling and angiogenesis [30]. Movement of endothelial cells within the corneal stroma that composed of mainly collagen fibrils requires proteolysis of the basement membrane and surrounding ECM [2].
Platelet derived growth factor (PDGF) is another important angiogenic factor that involved in maturation of nascent vessels. It ensures recruitment of pericyte and smooth muscle cells to endothelial cells [31]. Lots of cytokines, chemokines and adhesion molecules are also involved in angiogenesis [2].
B. Antiangiogenic Factors
Antiangiogenic factors mainly synthesized from corneal epithelium [2]. De-epithelialization of the mouse cornea promotes CorNV [32].
Angiostatin is proteolytic fragment of plasminogen [33]. It inhibits proliferation and migration of vascular endothelial cells [34]. Endostatin is another antiangiogenic factor that is a proteolytic fragment of collagen XVIII. It is activated by MMPs and interferes with endothelial cell proliferation [35]. Pigment epithelium derived factor (PEDF) is first identified in retinoblastoma cells. It is a potent antiangiogenic factor. Blocking antibodies against PEDF promote corneal vascularization when implanted in rat corneal stroma [36].
Soluble vascular endothelial growth factor receptor 1 (sVEGFR1) is a soluble form of VEGFR1 which acts as endogenous VEGF trap [37].
Clinical Examples of CorNV
Mainly, two clinical entities of corneal NV can be discerned: stromal or deep neovascularization and superficial neovascularization or vascular pannus. There is a correlation between etiological factors and depth of CorNV. For example, stromal keratitis is generally causing deep vascularization whereas ocular surface disorders and contact lens usage are associated with superficial vascularization [38].
Figure 1 and 3 show examples of stromal neovascularization associated with infectious etiologies. Figure 2 shows superficial neovascularization associated with limbal stem stem cell deficiency secondary to keratitis-ichthyosis-deafness (KID) syndrome.
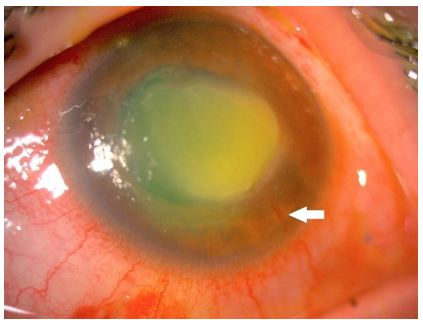
Figure 1: Anterior segment photograph of the stromal CorNV associated with Acanthamoeba keratitis.
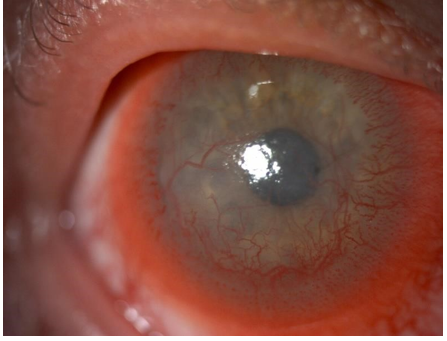
Figure 2: Anterior segment photograph of superficial CorNV associated with Keratitis ichthyosis-deafness (KID) syndrome.
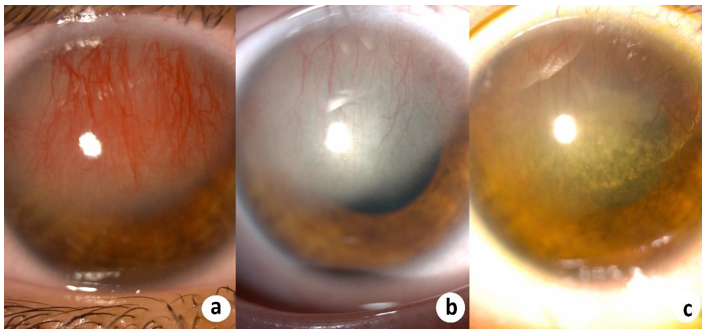
Figure 3: (a) Stromal CorNV secondary to herpetic keratitis. Regression of CorNV after (b) a week and (c) a month of the the anti-viral and corticosteroid treatment.
Treatment Options of CorNV
CorNV is often accompanied by decreased visual acuity. There are medical and surgical treatment modalities [27].
Topical corticosteroid agents have been the mainstay of CorNV treatment. Both anti-inflammatory properties and direct inhibition effects on vascular endothelial cell migration and proliferation are anti-angiogenic effects of corticosteroids [39]. Steroids are most effective in inhibiting CorNV when started early stages after tissue injury [2]. Non-steroidal anti inflammatory drugs (NSAID) have limited antiangiogenic effects [40].
Commercially available VEGF-A inhibitors such as bevacizumab, aflibercept and ranibizumab have been used in treatment of CorNV [40]. Aflibercept has dual effect in angiogenesis by both anti-VEGF effects and PDGF pathway blockage [41].
Medical treatment methods are relatively ineffective in the setting of mature vasculature. Surgical methods which used treatment of CorNV are physical ablation of vessels with argon laser and photodynamic therapy (PDT) [40]. Physical ablation with laser carries the risk of collateral damage to the surrounding cornea and limbal stem cells [2].
Conflict of interest
The authors declare that there is no conflict of interest.
Declaration:
Funding: The authors received no financial support for the research, authorship and publication of this article.
Declaration of Interest: The authors declare that there is no conflict of interest.
Authors’ Contributions: Mukaddes Damla Ciftci was responsible for conducting research, screening potentially eligible studies, drafting manuscript. Ozlem Barut Selver was responsible for coordinating the study, interpreting results and providing feedback on the final report. All authors read and approved the final manuscript.
References
- Isner JM, Asahara T. (1999) Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 103(9): 1231-1236. [PubMed.]
- Nicholas MP, Mysore N. (2021) Corneal neovascularization. Exp Eye Res. 202: 108363. [PubMed.]
- Gonzalez L, Loza RJ, Han KY, et al. (2013) Nanotechnology in corneal neovascularization therapy--a review. J Ocul Pharmacol Ther. 29(2): 124-134. [Ref.]
- Sridhar MS. (2018) Anatomy of cornea and ocular surface. Indian J Ophthalmol. 66(2): 190-194. [PubMed.]
- Sabatino F, Di Zazzo A, De Simone L, Bonini S. (2017) The Intriguing Role of Neuropeptides at the Ocular Surface. Ocul Surf. 15(1): 2-14. [PubMed.]
- Beebe DC. (2008) Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 19(2): 125-133. [Ref.]
- Conway EM, Collen D CP, Carmeliet P. (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001)49(3): 507-521. Cardiovasc Res. (49): 507-521. [Ref.]
- Djonov V, Baum O, Burri PH. (2003) Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 314(1): 107-117. [PubMed.]
- Di Zazzo A, Gaudenzi D, Yin J, et al. (2021) Corneal angiogenic privilege and its failure. Exp Eye Res. 204: 108457. [PubMed.]
- Lee P, Wang CC, Adamis AP. (1998) Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 43(3): 245-269. [PubMed.]
- Lasagni Vitar RM, Triolo G, Fonteyne P, et al. (2021) Epidemiology of Corneal Neovascularization and Its Impact on Visual Acuity and Sensitivity: A 14-Year Retrospective Study. Front Med (Lausanne). 8: 733538. [PubMed.]
- Robert PY, Liekfeld A, Metzner S, et al. (2006) Specific antibody production in herpes keratitis: intraocular inflammation and corneal neovascularisation as predicting factors. Graefes Arch Clin Exp Ophthalmol. 244(2): 210-215. [PubMed.]
- Tabatabaei SA, Soleimani M, Behrouz MJ. (2017) Torkashvand A, Anvari P, Yaseri M. A randomized clinical trial to evaluate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Ocul Surf. 15(2): 218-226. [Ref.]
- Lee MH, Wiedman G, Park S, Mustaev A, Zhao Y, et al. (2018) A novel, tomographic imaging probe for rapid diagnosis of fungal keratitis. Med Mycol. 56(7): 796-802. [Ref.]
- Kremer I, Cohen EJ, Eagle RC Jr, Udell I, Laibson PR. (1994) Histopathologic evaluation of stromal inflammation in Acanthamoeba keratitis. CLAO J. 20(1): 45-48. [Ref.]
- Kawashima M, Kawakita T, Satake Y, Higa K, Shimazaki J. (2007) Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch Ophthalmol. 125(10): 1337-1344. [PubMed.]
- Kılıç Müftüoğlu İ, Aydın Akova Y. (2016) Clinical Findings, Follow-up and Treatment Results in Patients with Ocular Rosacea. Turk J Ophthalmol. 46(1): 1-6. [Ref.]
- Power WJ, Tugal-Tutkun I, Foster CS. (1998) Long-term follow-up of patients with atopic keratoconjunctivitis. Ophthalmology. 105(4): 637-642. [Ref.]
- Mohammadpour M. (2007) Progressive corneal vascularization caused by graft-versus-host disease. Cornea. 26(2): 225-226. [Ref.]
- Skeens HM, Brooks BP, Holland EJ. (2011) Congenital aniridia variant: minimally abnormal irides with severe limbal stem cell deficiency. Ophthalmology. 118(7): 1260-1264. [Ref.]
- Van Acker SI, Haagdorens M, Roelant E, et al. (2019) Pterygium Pathology: A Prospective Case-Control Study on Tear Film Cytokine Levels. Mediators Inflamm. 2019: 9416262 [PubMed.]
- Sepehr F, (2017). Corneal angiogenesis: etiologies, complications, and management. Physiologic and pathologic angiogenesis - signaling mechanisms and targeted therapy. In: Physiologic and Pathologic Angiogenesis - Signaling Mechanisms and Targeted Therapy. [Ref.]
- Prabhasawat P, Tarinvorakup P, Tesavibul N, et al. (2005) Topical 0.002% mitomycin C for the treatment of conjunctival-corneal intraepithelial neoplasia and squamous cell carcinoma. Cornea. 24(4): 443-448. [Ref.]
- Cursiefen C, Rummelt C, Küchle M. (2000) Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 19(4): 526-533. [PubMed.]
- Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. (2001) Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 75(20): 9828-9835. [PubMed.]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, et al. (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 27(4): 331-371. [PubMed.]
- Chang JH, Gabison EE, Kato T, Azar DT. (2001) Corneal neovascularization. Curr Opin Ophthalmol. 12(4): 242-249. [PubMed.]
- Jiang J, Xia XB, Xu HZ, et al. (2009) Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1alpha and VEGF. J Cell Physiol. 218(1): 66-74. [Ref.]
- Clements JL, Dana R. (2011) Inflammatory corneal neovascularization: etiopathogenesis. Semin Ophthalmol. 26(4-5): 235-245. [PubMed.]
- Cui N, Hu M, Khalil RA. (2017) Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci. 147: 1-73. [PubMed.]
- Holmes DI, Zachary I. (2005) The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 6(2): 209. [PubMed.]
- Cursiefen C, Chen L, Saint-Geniez M, et al. (2006) Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 103(30): 11405-11410. [PubMed.]
- O'Reilly MS, Holmgren L, Shing Y, et al. (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 79(2): 315-328. [PubMed.]
- Cao R, Wu HL, Veitonmäki N, et al. (1999) Suppression of angiogenesis and tumor growth by the inhibitor K1-5 generated by plasmin-mediated proteolysis. Proc Natl Acad Sci U S A. 96(10): 5728-5733. [PubMed.]
- O'Reilly MS, Boehm T, Shing Y, et al. (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 88(2): 277-285. ' [PubMed.]
- Dawson DW, Volpert OV, Gillis P, et al. (1999) Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 285(5425): 245-248. [PubMed.]
- Rolfsen ML, Frisard NE, Stern EM, Foster TP, et al. (2013) Corneal neovascularization: a review of the molecular biology and curren. Expet Rev Ophthalmol. 8: 167-189. [Ref.]
- Cogan DG. (1962) Corneal neovascularization. Invest Ophthalmol Vis Sci. 1: 253-261. [PubMed.]
- BenEzra D, Griffin BW, Maftzir G, Sharif NA, Clark AF. (1997) Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Invest Ophthalmol Vis Sci. 38(10): 1954-1962. [PubMed.]
- Feizi S, Azari AA, Safapour S. (2017) Therapeutic approaches for corneal neovascularization. Eye Vis (Lond). 4: 28. [PubMed.]
- Jo N, Mailhos C, Ju M, et al. (2006) Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 168(6): 2036-2053. [PubMed.]