>Corresponding Author : Mark E Peacock
>Article Type : Research Article
>Volume : 5 | Issue : 2
>Received Date : 22 Oct, 2025
>Accepted Date : 03 Nov, 2025
>Published Date : 07 Nov, 2025
>DOI : https://doi.org/10.54289/JDOE2500110
>Citation : Price H, Ghaly M, and Peacock ME. (2025) Excessive Bleeding Associated with Chronic High-Dose Bitter Gourd Supplementation. J Dent Oral Epidemiol 5(2): doi https://doi.org/10.54289/JDOE2500110
>Copyright : © 2025 Price H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
Department of Periodontics, Dental College of Georgia at Augusta University, Augusta, Georgia
*Corresponding author: Mark E Peacock, Department of Periodontics, Dental College of Georgia at Augusta University, Augusta, Georgia
Abstract
Background: Herbal supplement use has been reported to be widespread and often not disclosed in patient medical history reviews. Too much supplementation and/or interaction(s) with other prescribed drug(s) can be a real danger and should always be investigated in the patient’s record. Some supplements have been described in the literature that could potentially affect bleeding and hemostasis during and after surgical procedures.
Methods and Results: This case describes a surgical patient who was taking daily supplemental bitter gourd powder and exhibited excessive bleeding intraoperatively, in addition to prolonged coagulopathy postsurgically. The postoperative hemorrhage did require additional intervention to help ensure stable clotting. Based on a scientific literature review, this is the first case of bleeding complications reported from excessive bitter gourd (melon) consumption in the dental/oral surgical/periodontal literature that could be found.
Conclusions: Clinicians should be aware of any herbal supplementation that a patient ingests, to include the amount/dosage of such medicinal intake. Any supplement usage that could possibly affect coagulation during/after surgery should be investigated, and the patient advised to stop the supplement (most supplements) for at least 2 weeks before the procedure. Consultation, if indicated, should always be pursued before treatment.
Keywords: Dietary Supplements, Surgical Bleeding, Herbal Medicine, Coagulopathy
Abbreviations: GI: Gastrointestinal, ER: Emergency Room, AAF: Active Anticoagulant Fraction
Introduction
Medicinal herb use from plant sources has been a practice that has been around for millennia. It has been reported that approximately a third of adult patients in the United States (US) take herbal supplements [1]. Rashrash et al, in a 2015 survey, disclosed that roughly 35% of US adults reported use of dietary herbal medications [2]. Skinner and Rangasami in a 2002 pre-anesthesia study in the United Kingdom (UK) found that 131 of 2723 patients were taking herbal medications, but in only 2 cases was this documented in the patients’ medical notes [3]. It is obviously apparent that many patients do not understand the potential risk(s) involved in not disclosing all medicines/supplements they may be taking. Thorough evaluation of a patient’s medical history, to include include all medicines and supplements they are consuming, is a mandatory preoperative step that must be completed [4].
Several studies have identified the most common herbal medications/dietary supplements that are encountered in patients scheduled for invasive procedures [1,3,5,6]. Hatfield et al recently reviewed dietary supplements that could potentially alter bleeding and coagulation during surgery, to include those ingested along with anticoagulants that the patient may have been prescribed [1]. Although many supplements are benign, they are not regulatory controlled like prescription drugs, and could lead to unidentified perioperative complications [7]. The two supplements that have shown to have the most evidence affecting surgical bleeding, independent of other prescribed anticoagulants, are garlic and hawthorn [1]. Several case reports have strongly associated garlic with excessive postoperative bleeding by means of platelet aggregation inhibition [8]. An evidence-based literature review by Malhotra et al in 2020 confirmed that high garlic consumption can predispose oral surgical patients to bleeding problems during and/or after the procedure, even though there was no protocol in the reviewed studies that assessed garlic intake during the preoperative evaluation [9].
Bitter gourd or bitter melon, an herbal fruit originally from Africa, but also observed in Asia, India, and Pakistan, was traditionally (complementary medicine) used for treating hypertension and diabetes. Despite widespread use of bitter gourd as a supplement (fruit, liquid, powder capsule form), there is a lack of documentation in the literature on the potential toxicity of this herb [10]. There are multiple reports of emergent conditions associated with bitter gourd, some fatal, in the scientific literature [10-17]. In all of these cases, the patients presented to emergency rooms with signs/symptoms of nausea, vomiting, diarrhea, hematemesis, upper gastrointestinal (GI) ulceration/bleeding, and dehydration. More than half of the patients were also hypotensive. Only one of the reports occurred in the US [14]. It is clear in these cases that the severe upper GI bleeding was a major concern in stabilization of the patients. Several fairly recent studies demonstrate the anticoagulant effects of bitter gourd and/or its fruit/seed extract [18-22]. Based on these recent scientific findings, it becomes apparent that besides the reported beneficial effects of this herb, there could also be possible severe adverse effects from overconsumption of bitter gourd. There is limited information available on possible perioperative consequences, and none in the dental/oral literature. This case describes a patient on elective bitter gourd supplement, who presented with unusually unaccountable excessive hemorrhage during and after routine dentoalveolar surgery.
Materials, Methods, and Results
The patient was referred and treated at the Department of Periodontics, the Dental College of Georgia (DCG) at Augusta University (AU), Augusta, Georgia. The patient electronically signed and gave verbal/written consent for examination/treatment, to include the use of clinical, radiographic, and photographic data for educational/research purposes.
A 56-year old male presented for exam/treatment of periodontitis and extraction of nonrestorable teeth. A review of the medical history revealed herniated disc, familial hyperlipidemia, and “borderline” hypertension (elevated, not staged). Current medications included hydrocodone/acetaminophen as needed, evolocumab, multivitamins, and fish oil. He also reported taking daily supplements of herbal bitter gourd for past 2-3 years. There were no known drug allergies. A comprehensive exam revealed that the patient had previously been treated for periodontal disease with successful initial nonsurgical debridement, and was placed on a periodontal maintenance plan. Teeth #s 28, 29, 30, and 31 were carious and nonrestorable. The surgical plan was to extract mandibular right posterior teeth utilizing local anesthesia. Anesthesia was obtained with topical 20% benzocaine plus 2% lidocaine with 1:100,000 epinephrine, 4% articaine with 1:100,000 epinephrine, and 0.5% bupivacaine with 1:200,000 epinephrine. A facial sulcular incision was made opposite #s 28-31 distal and a mucoperiosteal flap was elevated to facilitate sectioning of the brittle, carious teeth and alveolectomy/alveoloplasty. During the procedure and after teeth were removed, more than abnormal bleeding was observed, and no obvious single source of the bleed could be located. 2% lidocaine with 1:50,000 epinephrine was used to slow bleeding but was ineffective. Absorbable collagen was placed with pressure, but was unsuccessful. Laser-mediated hemostasis was attempted, but to no avail. Bone wax was placed at all hemorrhage bony sites with good hemostasis. Plugs of absorbable collagen were packed around the bone wax, pressure applied, and the sites were closed with 5-O chromic gut sutures. Good hemostasis was ensured and postoperative instructions given before the patient was dismissed. Later that evening, patient experienced additional bleeding and he went to the local emergency room (ER) (patient lives out-of-town). ER staff observed that the surgical sites were closed. Direct pressure was placed with moist gauze and good hemostasis was attained. Patient reported that for a “few days” after the ER visit, he experienced some bleeding (oozing) which was controlled by biting down on gauze. At the 2-week postoperative visit, sutures were absent and the sites has some lingual soft tissue ulceration, but no exposure of bone. The patient’s physician discussed stopping bitter gourd supplements for at least 2 weeks before any future surgery, and he agreed. Patient will be monitored before any future planned surgery.
Discussion
Bitter gourd (melon) is an herbal plant of the Cucurbitaceae (gourd) family that traditionally was consumed as food, and has purported medicinal value [23]. Also called cucurbits, others related to the gourd family are squash, pumpkin, cucumber, and zucchini. Cucurbitacin is a chemical compound found in the fruit of bitter gourd that is naturally produced as a defense against plant eating animals. There are many subtypes of cucurbitacin, with subtype D being very potent in increasing vascular (capillary) permeability [11]. In high doses this herbal compound can be dangerously toxic, even life threatening. Bitter gourd has been shown to have extremely high levels of cucurbitacins compared to others in the gourd family [14]. There is no treatment to counter the toxicity of bitter gourd.
Hemostasis in humans is a multifaceted process that leads to coagulation. After damaged vessels initially contract, primary hemostasis begins with platelet activation, followed by secondary hemostasis with the coagulation cascade, and finally leading to a stable fibrin clot. Figure 1 is an oversimplified diagram of the process. There are several reports in the literature by Manjappa et al looking at bitter gourd’s anticoagulant effect(s) [18,20,21]. A 2015 study showed that Momordica charantia (Mc) seed extract interfered with the intrinsic pathway of the cascade, and hydrolyzed the fibrin clot [20]. In another 2019 paper they discovered the antiplatelet effects of Mc seed [21]. And finally these authors in 2022 published findings on Mc seed oil extract that showed a biphasic effect on plasma coagulation, in addition to strong antiplatelet and antioxidant activities [18]. Gogoi et al in 2020 published findings of a separated active anticoagulant fraction (AAF) from fruit extract of Mc, which revealed the in vitro anticoagulant activity was equivalent to heparin and warfarin [22]. A fairly recent report (2023) also showed that Mc fruit extract exhibited significant anticoagulant and thrombolytic activity [19].
Most reports in the literature of cases presenting to emergency medical treatment facilities where patients ingested bitter gourd, the form of gourd ingestion was liquid (bottle gourd juice, homemade concentrated liquid extract). Demmers et al postulated that Mc-based formulated supplements had better quality control and are produced under stricter standards than home-based preparations [24]. They established that as long as the daily dosage of bitter gourd products were lower than 6 g (grams) per day, they found no serious harm or side effects. The subject patient in this report stated that he prepared his own capsules (large, approximately 800 mg) using a powdered form of bitter gourd (melon), and that he took 3 of these capsules three times a day (morning, noon, night). This would amount to a daily dose of more than approximately 7 g per day. The typical recommended dosage for OTC bitter gourd supplements (liquid, powder, tablets, capsules) is approximately up to 1000 mg two to three times daily (3g/day) [25]. The patient had been taking this supplement regimen for the past 2-3 years. The patient’s cardiologist counseled the patient to not take any bitter gourd supplements for at least 2 weeks prior to any future invasive procedure. This 2-week hold recommendation is also referenced specifically for bitter melon in a 2021 Mayo Clinical Proceedings Consensus Statement by Cummings III et al [6]. The patient has recently reported that he is now contemplating, along with his physicians’ advice, to stop the bitter gourd permanently.
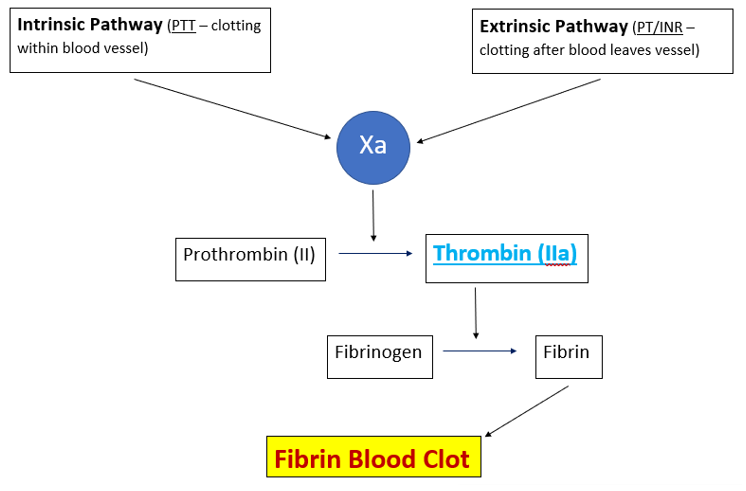
Figure 1: Basic coagulation overview
Conclusion
This case reinforces how vital it is for all clinical practitioners to perform an in-depth preoperative history review, to include asking patients of any history taking supplements and/or any other substances, legal or otherwise. Many times a patient may or may not volunteer this information unless the clinician specifically asks about it. When to restart a supplement after surgery, if the patient so chooses, should only be endorsed after there is minimal chance of any further postoperative hemorrhaging. It is imperative to remember, after an extensive review of the literature, that all supplements/substances that may alter coagulation have not been identified; and, are not listed in many peer-reviewed scientific documents on the subject of herbal supplements and bleeding risks(s).
Acknowledgments: The authors report no conflict of interest.
Patient Consent Statement: Treatment consent for the patient was obtained with oral permission and documented. There are no identifiers in the figure.
Summary
This case report documents a complication of excessive intraoperative and postoperative hemorrhage in an oral/periodontal surgical patient who had been taking high daily supplements of bitter gourd (melon) powder for several years. Practitioners need to be aware of potential side effects in patients taking herbal supplements that may affect coagulation.
References
- Hatfield J., Saad S., Housewright C. Dietary supplements and bleeding. Proc (Bayl Univ Med Cent). 2022;35(6):802-807. [PubMed.]
- Rashrash M., Schommer JC., Brown LM. Prevalence and predictors of herbal medicine use among adults in the United States. J Patient Exp. 2017;4(3):108-113. [PubMed.]
- Skinner CM., Rangasami J. Preoperative use of herbal medicines: a patient survey. Br J Anaesth. 2002;89:792-795. [PubMed.]
- Peacock ME., Carson RE. Frequency of self-reported medical conditions in periodontal patients. J Periodontol. 1995;66:1004-1007. [PubMed.]
- Ang-Lee MK., Moss J., Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286:208-216. [PubMed.]
- Cummings III KC., Keshock M., Ganesh R., et al. Preoperative management of surgical patients using dietary supplements: society for perioperative assessment and quality improvement (SPAQI) consensus statement. Mayo Clin Proc. 2021;96(5):1342-1355. [PubMed.]
- Ronis MJJ., Pedersen KB., Watt J. Adverse effects of nutraceuticals and dietary supplements. Annu Rev Pharmacol Toxicol. 2018;58:583-601. [PubMed.]
- Abebe W. Review of herbal medications with the potential to cause bleeding: dental implications, and risk prediction and prevention avenues. EPMA J. 2019;10(1):51-64. [PubMed.]
- Malhotra U., Hasday M., Romanos GE., Javed F. Assessment of routine diet (garlic consumption) as a pre- and postoperative protocol in oral and maxillofacial surgical interventions: an evidence-based literature review. Nutr Health. 2020;26(2):135-139. [PubMed.]
- Nadkarni N., D’Cruz S., Sachdev A. Hematemesis due to bitter melon (Momordica charantia) extract-induced gastric ulcerations. Indian J Gastroenterol. 2010;29(1):43-44. [PubMed.]
- Naysadorai KA., Bellary S. Bottle gourd (Lagenaria siceraria) toxicity: a rare case report. Clin Med. 2024;24:21-23. [Ref.]
- Sharma SK., Puri R., Toteja GS., et al. Assessment of effects on health due to consumption of bitter bottle bourd (Lagenaria siceraria) juice. Indian J Med Res. 2012;135:49-55. [PubMed.]
- Khatib KI., Borawake KS. Bottle gourd (Lagenaria siceraria) toxicity: a “bitter” diagnostic dilemma. J Clin Diag Res. 2014;8(12):5-7. [Ref.]
- Ho CH., Ho MG., Ho SP., Ho HH. Bitter bottle gourd (Lagenaria siceraria) toxicity. J Emerg Med. 2014;46(6);772-775. [PubMed.]
- Lee WT., Ng GG., Phua DH. Bottle gourd (Lagenaria siceraria) toxicity diagnosed in the emergency department. J Emerg Med. 2022;63(2):49-52. [PubMed.]
- Chavda DM., Parikh C., Patel VV., Pandya PS. Bitter bottle gourd poisoning: a case report and review of the literature. J Family Med Prim Care. 2022;11(7):4042-4044. [PubMed.]
- Verma A., Jaiswal S. Bottle gourd (Lagenaria siceraria) juice poisoning. World J Emerg Med. 2015;6(4):308-309. [PubMed.]
- Manjappa B., Sannaningaiah D. Oil extract of Momordica charantia seed (OEMCS) exhibits biphasic effect on blood coagulation., antiplatelet and antioxidant properties. Asian J Biol Life Sci. 2022;11(3):757-764. [Ref.]
- Azmi NC., Suhimi AA., Yahya TSANT. Anticoagulant evaluation of Momordica charantia fruit flesh extract on prothrombin time and activated partial prothrombin time test. Biomed & Pharmacol J. 2023;16(4):2205-2212. [Ref.]
- Manjappa B., Gangaraju S., Girish KS., et al. Momordica charantia seed extract exhibits strong anticoagulant effect by specifically interfering in intrinsic pathway of blood coagulation and dissolves fibrin clot. Blood Coagul Fibrinolysis. 2015;26(2):191-199. [PubMed.]
- Manjappa B., Shankar RL., Martin SS., Sannaningaiah D. Comparative evaluation of aqueous and ethanol extracts of Momordica charantia seed on coagulation cascade and platelet function. Int Res J Pharm. 2019;10(5):164-169. [Ref.]
- Gogoi D., Jha S., Chattopadhyay P. A simple, cost-effective., and rapid separation process for the isolation of anticoagulant active fraction from the fruit extract of Momordica charantia: characterization of bioactive components and anticoagulant mechanism of active fraction in a mouse model. J Sep Sci. 2020;43:3902-3912. [PubMed.]
- Ahmad N., Hasan N., Ahmad Z., Zishan M., Zohrameena S. Momordica charantia: for traditional uses and pharmacological actions. J Drug Deliv Ther. 2016;6(2):40-44. [Ref.]
- Demmers A., Mes JJ., Elbers RG., Pieters RHH. Possible harms of Momordica charantia L in humans; a systematic review. medRxiv. 2022;10.22.22281390. [Ref.]
- Liver tox: clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases;2012-Bitter Melon. 2023. [PubMed.]