>Corresponding Author : Mark E Peacock
>Article Type : Case Series
>Volume : 5 | Issue : 1
>Received Date : 01 July, 2025
>Accepted Date : 11 July, 2025
>Published Date : 17 July, 2025
>DOI : https://doi.org/10.54289/JDOE2500105
>Citation : Merchant S, Timothius CJC, Meghil MM, Ghaly M, Peacock MK, et al. (2025) Stabilization of Free Soft Tissue Grafts Utilizing Biocompatible Cyanoacrylate: Case Series Reports. J Dent Oral Epidemiol 5(1): doi https://doi.org/10.54289/JDOE2500105
>Copyright : © 2025 Merchant S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Series | Open Access | Full Text
1Private Practice, Chicago, Illinois
2Department of Periodontics, Dental College of Georgia at Augusta University, Augusta, Georgia
3Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia at Augusta University, Augusta, Georgia; Division of Clinical Dentistry, University of Detroit Mercy School of Dentistry, Detroit, Michigan
*Corresponding author: Mark E Peacock, Dental College of Georgia at Augusta University, Augusta, Georgia
Abstract
Background: Gingival recession and a lack of keratinized tissue are implicated in the progression of periodontal tissue destruction and can ultimately contribute to tooth loss. Various techniques and materials have been used in current practice, of which free gingival grafts (FGG) have revealed predictable outcomes in modifying gingival biotype and increasing the width of keratinized tissue. Stabilization of the donor graft, traditionally achieved with sutures, is a crucial factor in successful tissue augmentation. These reports detail an alternative approach of using a tissue adhesive (cyanoacrylate) to obtain similar outcomes with improved benefits.
Methods and Results: Two cases are presented illustrating the successful use of cyanoacrylate in graft stabilization and soft tissue adaptation. A well-designed technique is described to achieve outcomes with increased predictability and reproducible results. Usage of this material may avoid some of the potential negative consequences of suturing, such as donor tissue trauma and inflammation caused by suture materials. Cyanoacrylate has also been shown to result in reduced graft shrinkage.
Conclusion: Cyanoacrylate adhesives utilized instead of sutures may have a positive impact on patient comfort, reduced surgical chair time, and superior tissue adaptation, while also aiding in coagulation when used as a hemostatic agent at the graft donor site.
Keywords: Soft Tissue Grafts, Cyanoacrylate Tissue Adhesives, Gingival Augmentation
Abbreviations: FGG: Free Gingival Grafts, CA: Cyanoacrylate, FDA: Food and Drug Administration, DCG: Dental College of Georgia, AU: Augusta University, GERD: Gastroesophageal Reflux Disease
Introduction
A goal in successful soft tissue grafting is to achieve expedient and uncomplicated healing. Successful practitioners are constantly searching for techniques and procedures that achieve these surgical principles in a predictable way. Cyanoacrylate (CA) adhesive has been used in medicine/dental medicine for wound closure, hemostasis, fractures, and surgical procedures in multiple specialties, [1-3]. CA was first synthesized and patented by Ardis in 1949, [4] but not until 1959 did Coover create a CA product (Super Glue) that was used in medicine for wound closure, [5]. During the Vietnam War in the 1960’s a CA spray (Histoacryl Blue) was successfully tested and used by battlefield medics to stop severe bleeding, and Bhaskar in 1966 presented this adhesive to a conference at Walter Reed Army Medical Center to be used in periodontal and oral surgery, [6]. While the first tissue adhesives sometimes caused excessive inflammatory effects, there was a strong improvement in the material in the 1970s, [7]. In 1998 the Food and Drug Administration (FDA) authorized the use of 2-octyl cyanoacrylate for surgical use in skin closure (Dermabond).
A primary requirement of incision closure should include a technique that is rapid, relatively cost-effective, painless, and possibly bacteriostatic with optimal esthetic outcomes, [8]. The use of CA addresses many of these prerequisites, as it acts as a protective barrier which assists in wound healing, shortens operating time, eliminates the risks of sharps exposure via needle sticks, and results in esthetic benefits, [9]. Dental medicine applications of CA include gingival/mucosal wound closure, fixation of graft materials in regenerative procedures, oroantral communication closure, dental and maxillofacial fracture trauma, hemostasis, and endodontic surgery, [10]. The superiority of CA to adhere to moist tissues is a major advantage in utilizing it on oral mucosa. Recent data suggests that CA adhesives minimize inflammatory reactions when compared to conventional suturing, [11]. The use of CA in periodontal plastic surgical procedures such as FGGs can be dated back as early as 1968, documented in publications by Bhaskar and Frisch, [12,13]. The authors concluded that CAs have the ability to hold cut tissues together to promote healing, after which the material undergoes exfoliation, [13]. Soft tissue graft surgeries can be highly technique sensitive, and employing techniques and materials that yield predictable outcomes is in the benefit of the patient and surgeon. Applying a biocompatible CA to stabilize the graft produces advantages, such as minimizing trauma to the tissue and reducing surgical time for the operator and patient. This case series demonstrates the technique for successful use of CA tissue adhesive for graft stability.
Materials, Methods, and Results
Patients were referred and treated at the Department of Periodontics, the Dental College of Georgia (DCG) at Augusta University (AU), Augusta, Georgia. The patient or parent/guardian electronically signed and gave verbal/written consent for examination/treatment, to include the use of clinical, radiographic, and photographic date for educational/research purposes.
Case 1: A 75-year-old Caucasian female presented for exam/treatment of progressive gingival recession at the mandibular incisors. A review of the medical history reveals hypertension, history of pulmonary embolism, and gastroesophageal reflux disease (GERD). Current medications included metoprolol, apixaban, and lansoprazole. There were no known drug allergies. Per medical consultation with patient’s physician, apixaban was stopped 3 days prior to surgery and restarted 2 days after. (Figure 1A) shows a facial view of the mandibular anterior teeth with open interproximal contacts at #s 23-25 and minimal attached gingiva at central incisors. The surgical plan was to retain the minimal keratinized tissue present, and to augment gingiva with a free tissue graft. Local anesthesia was obtained with topical 20% benzocaine and 2% lidocaine with 1:100,000 epinephrine. An incision was made at the facial mucogingival junction, careful to retain minimal gingiva present, and a periosteal recipient bed was created for the graft (Figure 1B). A FGG was obtained from the donor palate and good hemostasis was obtained. The graft was placed at the recipient site and stabilized with CA (Figure 1C). Healing was uneventful, as seen in (Figures 1D) (1 month postop) and 1E (3 month postop). Careful application of the CA material (Periacryl 90, GluStitch) onto the graft is important; the following describes briefly the technique, as illustrated in (Figure 2). After placement of the graft, a pipette tip was used to dispense the adhesive in minor increments, ensuring that none of the CA becomes displaced beneath the graft. The CA is dispersed slowly going from a direction of tooth to graft. Sterile water was applied over the CA/graft, followed by immediate digital pressure in order to avoid any space/hematoma inferior to the graft. In the event that CA comes in between the graft and recipient bed; the stabilization procedure should be immediately repeated with new CA application. Leaving any unwanted material between recipient bed and graft could result in graft necrosis.

Figure: 1 A

Figure: 1 B

Figure: 1 C

Figure: 1 D

Figure: 1 E
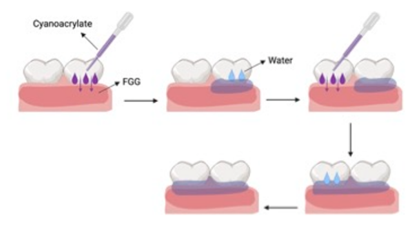
Figure: 2
Case 2: A 15-year-old Caucasian male was referred to the Periodontics Department from the DCG at AU Department of Orthodontics for evaluation/treatment of very thin keratinized tissue at facial of mandibular anteriors before initiating orthodontic treatment (Figure 3A). Patient’s medical history was unremarkable, no current medications taken and no known drug allergies. The approved surgical plan was to augment the minimally thin keratinized tissue before initiation of therapeutic tooth movement. Local anesthesia consisted of 20% topical benzocaine and 2% lidocaine with 1:100,000 epinephrine. Incisions were made to prepare the recipient periosteal bed (Figure 3B) and harvest the donor tissue from the palate (Figure 3C). The FGG was stabilized using the same method as described in the previous Case 1 (Figure 3D), (Figures 3E) (residual CA still in place) and (Figure 3F) (immediately after removal of CA adhesive) depict the graft surgical site at the 2-week postop appointment.
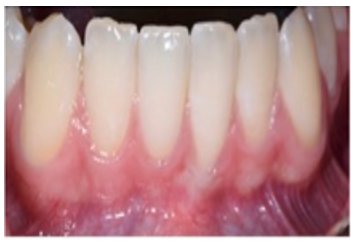
Figure: 3 A
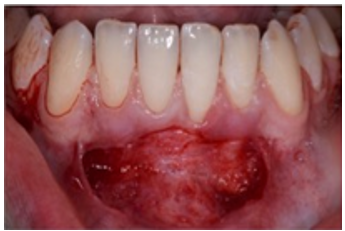
Figure: 3 B
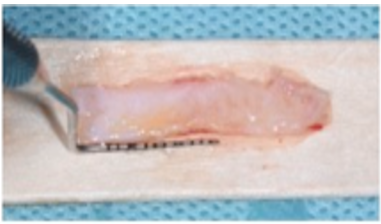
Figure: 3 C

Figure: 3 D

Figure: 3 E

Figure: 3 F
Discussion
Lang and Loe published over 50 years ago (1972) the landmark study showing that when plaque control was ideal, patients could accept a minimal amount of keratinized tissue/attached gingiva, and do very well, [14]. However, most patients do not possess perfect oral hygiene, and the authors revealed that usually a minimal width of 2 mm keratinized tissue was necessary to maintain periodontal health. Malpositioning and/or a thin gingival phenotype could exacerbate the issue with less-than-optimal hygiene. Increasing the zone of attached gingiva to help prevent future recession is a common surgical modality of most periodontists, and stabilizing these soft tissue grafts is of the utmost importance in revascularization/survivability of the graft. As evidenced by the two cases in this report, using a CA tissue adhesive instead of sutures can have many benefits for both operator and patient. Good tissue adaptation while minimizing graft trauma from needles and less surgical chair time are desirable goals. Estrin et al recently documented 111 consecutive patients treated for gingival augmentation around teeth and implants, all successfully stabilized using medical grade CA without any sutures, [15]. Multiple studies also report less perceived postoperative pain when using CA instead of sutures, [16-18]. The advantage of shortening operative time without losing clinical efficacy could also be valuable in treating patients with extensive pre-existing medical conditions.
When suturing soft tissue grafts, practitioners have to consider advantages and disadvantages of each type of material. Many dental surgeons consider a monofilament suture, such as nylon, to have advantages over braided multifilament materials like Polyglactin 910, when doing soft tissue procedures. There is less capillarity (capillary action) with monofilament materials, however cut ends of nylon can be sharp and cause soft tissue irritation. Capillarity has been shown to be have negative wound healing effects, although this consequence can be reduced by “coating” the suture material. Potential disadvantages when using sutures can be eliminated by stabilizing the wound/graft with CA.
Proper handling when working with CA is important to avoid any potential toxic hazards, such as contact dermatitis, urticaria, thermal release, and/or initiation of asthmatic reaction in susceptible individuals. The early CA monomers possessed short side chain derivatives, which increased the potential harmful effects. These formulations are no longer used, and the ones in medical/dental practice today (longer alkyl chain length, like butyl- and octyl- CA) are much safer with less toxicity. With proper use of PPE (personal protective equipment) and infection control standards in practice today, handling CA is considered much more innocuous. While CA material is considered “user friendly,”as with any clinical procedure, case selection is always of the utmost importance.
Conclusion
As evidenced by the cases presented, the use of CA to stabilize donor tissue at a recipient surgical site illustrates many benefits, to include minimizing needle trauma to graft, improved surgical time, possible cost effectiveness, harmonious tissue adaptation, hemostatic advantages, and increased patient comfort. With proper case selection, CA should be considered a viable alternative to using conventional treatments in oral surgery/periodontal patients. Long term comparative clinical studies should be considered moving forward.
Acknowledgments: The authors report no conflict of interest.
Patient Consent Statement: Treatment consent for each patient was obtained with oral permission. There are no identifiers in the figures.
References
- Borie E., Rosas E., Kuramochi G., Etcheberry S., Olate S., Weber B. Oral applications of cyanoacrylate adhesives: a literature review. BioMed Res Int. 2019;1-6. [PubMed.]
- Krol M., Kolasinska J., Maciaszczyk S., Nijakowski K. Cyanoacrylate glue application in oral surgery: a mini review. J Stoma. 2025;78(1):75-81. [Ref.]
- Cerda DG., Ballester AM., Aliena-Valero A., Caraben-Redano A., Lloris JM. Use of cyanoacrylate adhesives in general surgery. Surg Today. 2015;45(8):939-956. [PubMed.]
- Ardis AE. US patent no 2467926 and 2467927. 1949. [Ref.]
- Coover HW., Joyner FB., Shearer NA., et al. Chemistry and performance of cyanoacrylate adhesives. J Soc Plast Surg Eng. 1959;15:5-6. [Ref.]
- Bhaskar SN., Frisch J., Margetis PM., Leonard F. Oral surgery-oral pathology conference no 18, Walter Reed Army Medical Center. Application of a new chemical adhesive in periodontic and oral surgery. Oral Surg Oral Med Oral Pathol. 1966;22(4):526-535. [PubMed.]
- Leggat PA., Smith DR., Kedjarune U. Surgical applications of cyanoacrylate adhesives: a review of toxicity. ANZ J Surg. 2007;77(4):209-213. [PubMed.]
- Zuhr O., Akakpo DL., Hurzeler M. Wound closure and wound healing. suture techniques in contemporary periodontal and implant surgery: interactions, requirements, and practical considerations. Quintessence Int. 2017;647-660. [PubMed.]
- Grisdale J. The use of cyanoacrylates in periodontal therapy. J Can Dent Assoc. 1998;64(9):223-233. [PubMed.]
- Miranda M., Gianfreda F., Molica G., Martelli M., Gargari M., Bollero P. The use of cyanoacrylate and glubran in dentistry: a review of clinical applications and outcomes. Materials. 2025;18(11):2642. [PubMed.]
- AlJasser RN., AlSarhan MA., AlOtaibi DH et al. Comparison of polymeric cyanoacrylate adhesives with suturing in free gingival graft stability: a split mouth trial. Polymers. 2021;13(20):3575. [PubMed.]
- Bhaskar SN., Frisch J. Use of cyanoacrylate adhesives in dentistry. J Am Dent Assoc. 1968;77(4):831-837. [PubMed.]
- Frisch J., Bhaskar SN. Free mucosal graft with tissue adhesives: report of 17 cases. J Periodontol. 1968;39(4):190-195. [PubMed.]
- Lang NP., Loe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623-627. [PubMed.]
- Estrin NE., Kanter MS., Miron RJ. A simplified sutureless free gingival graft: a case series of over 100 consecutive patients. Int J Periodontics Restorative Dent. 2025;1-25. [PubMed] [PubMed.]
- Gumus P., Buduneli E. Graft stabilization with cyanoacrylate decreases shrinkage of free gingival grafts. Aus Dent J. 2014;59(1):57-64. [PubMed.]
- Alhourani MA., Kasem T., Hamadah O. Comparative study between using a tissue adhesive (N-BCA & OCA) and surgical sutures in free gingival graft surgery: a randomized controlledclinical trial. Dent Med Probl. 2022;59(2):241-248. [PubMed] [PubMed.]
- Gautam J., Sood A., Chaudhry S et al. Beyond the needle: a comparative evaluation of silk sutures and cyanoacrylate for periodontal flap closure. Cureus. 2024;16(3): e56604. [PubMed.]