>Corresponding Author : Hamdi Hamama
>Article Type : Review Article
>Volume : 5 | Issue : 1
>Received Date : 06 June, 2025
>Accepted Date : 20 June, 2025
>Published Date : 24 June, 2025
>DOI : https://doi.org/10.54289/JDOE2500104
>Citation : Elsherbiny N, Ebaya M, Hamama H. (2025) Topic: Recent Advances in Antibacterial Dental Adhesives. J Dent Oral Epidemiol 5(1): doi https://doi.org/10.54289/JDOE2500104
>Copyright : © 2025 Elsherbiny N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Review Article | Open Access | Full Text
Conservative Dentistry Department, Faculty of Dentistry, Mansoura University, Egypt
*Corresponding author: Hamdi Hamama, Clinical Professor, Conservative Dentistry Department, Faculty of Dentistry, Mansoura University, Mansoura, Egypt
Abstract
Background: Bonded restorations have transformed modern dentistry by enabling minimally invasive, esthetic, and durable tooth restorations. However, the long-term effectiveness of dental adhesives is challenged by the complex nature of dentin bonding, hydrolytic and enzymatic degradation, and bacterial infiltration at the adhesive interface, which contribute to restoration failure and secondary caries.
Objectives: This review aims to summarize recent advances in antibacterial dental adhesives, focusing on the incorporation of bioactive and nanostructured additives—particularly chitosan and graphene-oxide—to enhance bond durability, mechanical properties, and antibacterial performance.
Methods: A comprehensive analysis of contemporary literature was conducted, evaluating the mechanisms of adhesive degradation, the challenges of dentin bonding, and the efficacy of various additives. The review synthesizes findings regarding the chemical, mechanical, and biological effects of incorporating chitosan and graphene-oxide into dental adhesives, as well as the impact of nanoparticles and solvent systems on adhesive performance.
Conclusion: Recent advances in antibacterial dental adhesives, particularly through the incorporation of chitosan and graphene-oxide, have significantly improved the durability, mechanical strength, and antibacterial properties of dental restorations. These bioactive and nanostructured additives address key challenges in dentin bonding, such as hydrolytic and enzymatic degradation and bacterial infiltration, thereby enhancing restoration longevity. However, limitations remain regarding optimal additive concentrations, potential effects on esthetics, viscosity, and polymerization, as well as the need for more strong clinical validation. Ongoing research is essential to optimize formulations and confirm long-term clinical benefits.
Keywords: Challenges in Dentin Bonding; Antibacterial and Bioactive Additives; Nanoparticle Incorporation; Limitations
Abbreviations: MMPS: Matrix Metalloproteinases, CA: Calcium, P: Phosphorus, HEMA: Hydroxyethyl Methacrylate, CHX: Chlorhexidine, UDAS: Universal Dental Adhesives, MDP: Methacryloyloxydecyl Dihydrogen Phosphate, PY: Pyrogallol, TIO2: Titanium Dioxide, AGNPS: Sanguinis. Silver Nanoparticles, SI-NHAP: Silica-Doped Nanohydroxyapatite, ZNO NPS: Zinc Oxide Nanoparticles, SBS: Shear Bond Strength, CS-NPS: Chitosan Nanoparticles, CQ: Camphorquinone, ARI: Adhesive Remnant Index, CGIC: Conventional Glass Ionomer Cement, RMGIC: Resin- Modified Glass Ionomer Cement
Review
Bonded restorations have transformed modern dentistry by offering minimally invasive, esthetic and durable solutions for tooth restoration. The concept of bonded restorations relies on adhesive technology, enabling restorations to adhere securely to tooth structures without need for extensive preparation [1]. The typical procedure involves etching the tooth surface to create micro porosities in enamel, followed by application of an adhesive resin that penetrates the pores to create a strong mechanical bond. The interaction between the bonding agent and the tooth structure forms a bond that resists the forces exerted on the restoration [2,3].
Dental adhesives encounter significant challenges in maintaining long-term effectiveness, particulary due to the complex nature of bonding to dentin. Unlike enamel, dentin is a water-rich, organic substrate composed mainly of a collagen matrix and characterized by a tubular structure, which complicates durable adhesion. The intrinsic moisture and organic content lead to difficulties in achieving a stable resin-dentin bond. The resin-dentin interface is compromised by hydrolytic degradation and the enzymatic breakdown of exposed collagen fibrils by matrix metalloproteinases (MMPs), proteolytic enzymes that reside within the mineralized dentin matrix, [4,5]. Matrix metalloproteinases such as MMP-2 and MMP-9 become activated during the bonding process, especially after acid etching or self-etching procedures, which lower the pH and release these enzymes from their inactive proenzyme forms. Once activated, MMPs degrade the collagen fibrils in the hybrid layer, weakening the bond over time [6].
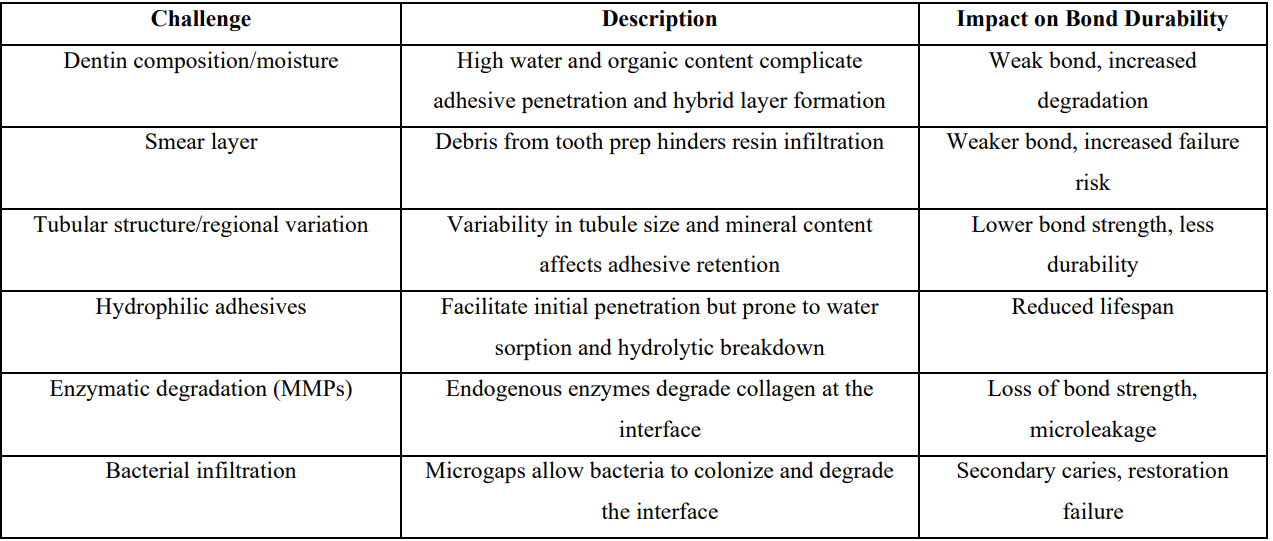
Bacterial infiltration at the dentin-adhesive interface is a critical factor in development of secondary caries and restoration failure. Imperfect bonding between resin and dentin results in microgaps that allow oral bacteria to penetrate and colonize the interface. This bacterial invasion leads to recurrent or secondary caries around restorations, a primary cause of restoration failure and replacement, accounting for a significant proportion of restorative dental procedures. The presence of bacteria and their metabolic byproducts further undermines the integrity of the adhesive interface by promoting acid-induced degradation and enzymatic breakdown of the exposed collagen matrix. Over time, these processes weaken dentin-adhesive bond, resulting in marginal breakdown, loss of seal, and ultimately restoration failure. This bacterial challenge, combined with hydrolytic and enzymatic degradation, underscores the difficulty in achieving a long-lasting, durable bond to dentin [7].
Bioactive and nanostructured additives such as chitosan and graphene-oxide have been incorporated into dental adhesives. When chitosan is added to universal adhesives, it interacts with dentin collagen, stabilizing the dentin matrix, inhibiting MMPs, and reducing degradation of the adhesive interface, thereby enhancing bond durability [8]. Additionally, Chitosan decreases dentin permeability and hypersensitivity by forming a calcium phosphate layer on demineralized dentin, promoting remineralization and protecting the adhesive interface over time [9]. As biocompatible and biodegradable biopolymer, chitosan is valued for its antimicrobial, anti-inflammatory, and remineralization properties [8].
Adhesives by increasing shear bond strength and improving adhesion to tooth structures. Its two-dimensional reduce the risk of secondary caries by inhibiting bacterial colonization at the tooth-restoration interface [11]. Additionally, the incorporation of Graphene-oxide has been associated with reduced nanoleakage and improved resistance to moisture, which are critical for the long-term durability of adhesive restorations [10,12]. micromechanical interlocking within the hybrid layer, resulting in stronger and more durable bonds [10]. Beyond mechanical reinforcement, Graphene-oxide imparts notable antibacterial properties to universal adhesives, helping to nanostructure, large surface area and hydrophilic nature allow for deeper penetration into the dentin and more effective
1.Benefits of adhesives restorations
Bonded restorations offer several significant advantages over traditional restorative methods. They allow preservation of more natural tooth structure due to minimal tooth preparation, thereby maintaining the integrity of the tooth [13,14]. Few studies [15-17] have shown that bonded restorations, whether using composite resin or bonded amalgam, provide significantly higher fracture resistance compared to non-bonded restorations. Additionally, bonded restorations, especially those using resin composites, deliver excellent esthetic results, as they can be color-matched to the surrounding tooth structure for a natural appearance that enhances patient satisfaction [14].
Tennert et al ,[18] and Tsujimoto et al, [14] reported that the longevity of direct bonded restorations in posterior teeth is comparable to that of indirect restorations, making them a reliable and durable option for long-term restorative needs. Bhargava et al,[19] showed that bonded restorations can reinforce vital tooth structure by maintaining integrity of teeth. Blatz [20]. concluded that adhesive dentistry facilitates tooth structure preservation and the use of highly esthetic materials through proper bonding protocols.
2. Bonding to dentin
In comparison with reliable enamel bonding, achieving a strong and predictable bond to dentin substrates remains more challenging and unpredictable [21,22]. As a result, establishing a durable bond to dentin is a critical aspect of restorative dentistry. The long-term success of adhesive restorations depends on the formation of a stable interface between dentin and the bonding agent. However, this process is complicated by the heterogeneous nature of dentin, the presence of a smear layer, variations in tubule density, and the risk of hydrolytic degradation all contribute to the difficulty of achieving a reliable bond.
2.1. Challenges in bonding to dentin
2.1.1. Dentin composition and moisture content
Dentin is composed of approximately 50% inorganic material, 30% organic matrix (mainly collagen) and 20% water, all of which affects adhesive penetration and bonding efficacy [23]. Moisture management is crucial during bonding, as the presence of water is essential for hybrid layer formation, However both excessive drying or over-wetting can compromise bond strength [24]. Achieving optimal moisture control is vital to maintain the structural integrity of the collagen-resin hybrid layer, which is essential for effective micromechanical retention. Additionally, residual water and acidic conditions in over-hydrated dentin can activate MMPs, enzymes that degrade collagen and compromise the resin-dentin interface over time [25].
2.1.2 Smear layer formation
The formation of a smear layer is one of the major challenges in achieving effective bonding to dentin. The smear layer is a thin, amorphous layer of debris created on the dentin surface during tooth preparation. It is composed of hydroxyapatite crystals, denatured collagen fibrils, saliva, blood and other debris, and it can extend into dentinal tubules forming smear plugs which reduce dentin permeability by up to 86% [26,27]. The smear layer can also harbor MMPs and other enzymes that degrade collagen fibrils, further compromising bond durability [7,26]. Accordingly dense or thick smear layer hinders resin infiltration, leading to weaker bonding and increased risk of adhesive failure [27]. Some bonding systems require complete removal of the smear layer, others rely on modifying it for effective adhesion [28]. Therfore managing the smear layer either by complete removal, modification, or controlled incorporation is critical for achieving strong, durable dentin bonding. Various strategies, including chemical agents to deproteinize or dissolve the smear layer and optimized adhesive application techniques, are used to mitigate its negative effects and improve bonding outcomes [27].
2.1.3. Tubular Structure and regional variability
Dentin is a highly dynamic biological tissue with a tubular structure that varies significantly depending on its location. Deep dentin exhibits large dentinal tubule diameter with increased fluid flow, which can dilute adhesives and reduce bond strength [29]. Additionally, sclerotic and carious dentin present further bonding challenges due to their altered mineral content and reduced permeability. Sclerotic dentin is characterized by reduced calcium (Ca) and phosphorus (P) content in superficial layers [30], tubular occlusion from mineral deposits that decreases permeability so these changes diminish micromechanical retention for adhesives [31]. Carious affected dentin, in contrast, is partially demineralized and contains a disrupted collagen matrix, making it less receptive to adhesive penetration and hybrid layer formation. These substrate variations often result in low bond strengths and reduced durability of the adhesive interface [32,33].
2.1.4. Hydrophilic nature of adhesives
Modern adhesive systems are designed to be hydrophilic to facilitate interaction with the moist dentin surface. This hydrophilicity often achieved through monomers like 2-hydroxyethyl methacrylate (HEMA), enables effective penetration into the collagen matrix of dentin, promoting micromechanical interlocking and chemical bonding essential for initial bond strength [34]. The presence of water and hydrophilic monomers enhances wetting and allows the adhesive to penetrate the moist substrate, which is critical since dentin is a hydrated biological composite. However, this characteristic also makes them prone to water sorption, leading to hydrolytic degradation of the adhesive resin over time. This hydrolytic degradation process compromises the structural integrity of the bond and reduce lifespan of the restoration [7]. To address these challenges, research has focused on balancing hydrophilicity for initial dentin infiltration with hydrophobicity to enhance long-term stability. Adhesives incorporating more hydrophobic monomers or those with reduced HEMA content demonstrate lower water sorption and better resistance to hydrolytic breakdown [34]. Increasing crosslink density and using monomers with higher hydrolytic stability are strategies to minimize water diffusion and improve the durability of the resin-dentin bond [5].
2.1.5. Enzymatic degradation
Dentin contains endogenous enzymes like matrix metalloproteinases (MMPs) and cysteine cathepsins that remain inactive within the mineralized matrix but become activated during adhesive procedures through acid etching, pH changes, and water exposure from bonding systems [35]. This enzymatic activation initiates collagen degradation at the resin-dentin interface, compromising the hybrid layer's structural integrity and contributing to bond strength reduction over time [36]. Pashley and Sabatini [37] highlighted that collagen fibrils exposed by acid etching become vulnerable to degradation by host-derived enzymes such as MMPs and cathepsins, which significantly reduce long-term bond strength. The hybrid layer, formed by resin monomer infiltration into the demineralized collagen network, is fundamental for the retention and sealing of adhesive restorations but is also the weakest link due to incomplete resin infiltration and the difficulty in displacing water trapped within collagen fibrils [5,7]. This incomplete hybridization leaves collagen vulnerable to enzymatic degradation and mechanical fatigue under masticatory forces [4]. Additionally, aqueous inclusions within the hybrid layer facilitate hydrolysis of the resin matrix and further activate endogenous enzymes such as MMPs and esterases, accelerating adhesive interface degradation [4,38].
To counteract this, inhibitors like chlorhexidine (CHX) and synthetic MMP inhibitors (e.g., galardin) have been applied to dentin surfaces or incorporated into adhesives to reduce enzymatic activity and preserve bond strength, demonstrating effectiveness in both laboratory and clinical settings [39,40]. Yaghmoor et al, [41] reported that incorporating MMP inhibitors like CHX into dental adhesives can increase immediate and long-term bond strength to dentin. While Breschi et al, [42] concluded that use of galardin helps preserve the hybrid layer and bond strength over time. Furthermore, cross-linking agents and adhesive modifications aim to stabilize collagen fibrils and reduce water permeability, enhancing the hybrid layer’s resistance to enzymatic attack [35]. Despite these strategies, enzymatic breakdown of collagen by activated proteases remains a major contributor to bond failure, resulting in loss of bond strength, microleakage, recurrent caries, and restoration failure over time [4,38]. Improving adhesive penetration, inhibiting endogenous proteases, and employing collagen cross-linkers continue to be critical approaches in efforts to achieve a durable, fully infiltrated hybrid layer that resists hydrolytic and enzymatic degradation, which remains a significant challenge for extending the clinical longevity of resin-based restorations [38]. Additionally, Albaladejo et al and Tran et al,[43,44] concluded that while etch-and-rinse adhesives tend to form thicker hybrid layers, self-etching systems may offer greater longevity by producing a more stable resin-dentin interface with less nanoleakage and degradation over time. Therefore, improving the quality and resistance of the hybrid layer remains a central focus in the development of more durable adhesive systems for restorative dentistry [5].
2.2. Bonding to caries-repairable dentin
Caries-affected dentin forms inner layer of dentin beneath bacterially infected dentin in a carious lesion. It is characterized by being demineralized and softened but not invaded by bacteria. Unlike infected dentin, caries-affected dentin retains an intact collagen matrix with some mineral content, giving it a firmer, leatherlike consistency. This dentin is partially demineralized but has the potential for remineralization and repair because its collagen fibrils remain mostly intact, making it a more reliable substrate for adhesive bonding compared to infected dentin [45].
Bonding to caries-repairable dentin poses significant challenges in restorative dentistry due to alterations in the dentin’s structural and chemical properties caused by the carious process. One major issue is the reduced bond strength compared to sound dentin, primarily because demineralization affects both the mineral and organic components, especially collagen. Partial denaturation and demineralization of the collagen matrix in caries-repairable dentin hinder resin monomer infiltration and stable hybrid layer formation, leading to compromised bond strength.
Additionally, caries-repairable dentin retains higher moisture content, which interferes with adhesive polymerization by diluting primers and inhibiting resin infiltration, further weakening the bond and reducing restoration longevity [46]. The heterogeneous structure of caries-repairable dentin-with variable degrees of demineralization and collagen degradation complicates uniform adhesive infiltration. This variability results in incomplete hybrid layers and increased risk of restoration failure, as the porous and irregular dentin surface challenges complicates adhesive application and hybrid layer formation [46,47]. Acid etching of caries-repairable dentin can reduce the number of effective bonding sites due to the altered mineral composition and the presence of mineral deposits within dentinal tubules that are resistant to removal by phosphoric acid. These mineral deposits limit resin monomer infiltration, resulting in incomplete hybrid layer formation and weaker adhesion. Additionally, the irregular and sclerotic nature of caries-repairable dentin means that conventional acid etching may not fully demineralize the substrate, reducing the available surface for bonding. Therefore, acid etching in caries-repairable dentin must be carefully controlled to avoid reducing bonding sites and weakening the adhesive interface [48,49].
Isolan et al, [50] found that bonding to sound dentin generally yields better results compared to caries-repairable dentin for both etch-and-rinse and self-etch adhesives. However, Nicoloso et al, [51] concluded that universal adhesives show similar bond strengths to caries-repairable dentin regardless of application mode. Mohanty et al, [45] showed that self-etch adhesives generally exhibit high bond strength to caries-repairable dentin compared to etch-and-rinse systems. Overall, bonding to caries-repairable dentin remains challenging, with most adhesives showing decreased bond strength compared to sound dentin [52].
3. Universal adhesive
The development of universal dental adhesives (UDAs) represents a significant advancement in adhesive dentistry, streamlining bonding protocols while maintaining high adhesive performance. Historically, dental adhesives evolved from multi- step etch-and-rinse systems to simplified self-etch systems, with UDAs marking the latest innovation by integrating both approaches into a single application [53]. These adhesives are formulated to adhere effectively to enamel, dentin, and restorative materials, making them compatible with both direct and indirect restorations. Traditional adhesive systems were classified into generations, each improving upon its predecessor by enhancing bond strength, reducing technique sensitivity, and improving moisture tolerance. The demand for simplified, yet effective adhesive protocols led to the development of universal adhesives, which aim to consolidate bonding procedures without compromising clinical performance [54].
Unlike conventional adhesives that require specific etching modes, UDAs can be applied using self-etch, selective enamel etch, or total-etch techniques, offering exceptional versatility in clinical applications [55]. This flexibility allows clinicians to tailor their bonding approach to individual patient needs, minimizing postoperative sensitivity and improving bond longevity. Furthermore, UDAs are designed for use with various restorative materials including composites, ceramics, and metals, making them a practical solution for modern restorative dentistry. A truly universal adhesive possesses several key attributes: Sufficient hydrophobicity to function as a single-layer adhesive, compatible with all etching modes, and suitable for use with any light-cure, dual-cure, or self-cure restorative material without the need for separate activators. Additionally, these adhesives exhibit low film thickness (typically less than 10µm), which is critical for achieving durable bonds with indirect restorations and minimizing interfacial gaps.
Chemically, UDAs commonly incorporate the adhesive monomer 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), which enables strong chemical bonding to hydroxyapatite in tooth structures as well as to restorative substrates such as zirconia and metals [56]. This chemical interaction contributes to the durability and stability of the adhesive interface [57]. The widespread adoption of UDAs is further supported by their compatibility with a variety of indirect restorations and their ability to bond to both dentin and enamel without additional primers or conditioners. This adaptability simplifies clinical workflows and reduces chairside time while maintaining bond integrity over extended periods [58].
Elkaffas et al, [59] showed that universal adhesives also known as multi-mode adhesives can achieve substantial bonding strength to dentin regardless of the application mode used, whether etch-and-rinse or self-etch. Nagarkar et al, [60]. Perdigão and Loguércio [61] reported that universal adhesives can be applied using self-etch, selective enamel etch, or total-etch techniques, providing flexibility in clinical applications. Breschi [62] concluded that UDAs are designed to bond effectively to various substrates, including enamel, dentin, and restorative materials. Rosa et al, [63] showed that although UDAs simplify procedures and reduce technique sensitivity, their performance may vary depending on the specific product and application mode. Studies [60,64,65] concluded that despite their advantages, UDAs still face challenges such as hydrolytic degradation and the need for long-term clinical data.
4. Enhancement of dental adhesive in composition according to their nature

4.1. Bioactive & Antimicrobial Additives
CHX is widely recognized for its dual ability to reduce bacterial colonization and enhance the durability of dental adhesive bonds. It achieves this by inhibiting MMPs, which play a key role in the degradation of the adhesive interface over time [66]. Pyrogallol (PY), a phenolic compound with strong antioxidant and antimicrobial properties, has also been shown to increase antibacterial activity and improve bond strength to dentin [67]. Additionally, incorporation of chitosan, a natural biopolymer with known antimicrobial activity, into dental adhesives can enhance their antibacterial properties, providing a synergistic effect when combined with other antimicrobial agents [68].
4.2. Nanoparticles
The incorporation of various nanoparticles into dental adhesives has significantly advanced their performance and longevity. Titanium dioxide (TiO₂) nanoparticles are particularly notable for enhancing mechanical properties such as flexural strength, hardness, wear resistance, and the degree of conversion. They also impart antibacterial activity and improve adhesive bond strength [69-71]. Sodagar et al, [72] and Hiers et al, [73] have demonstrated that TiO₂ nanoparticles provide significant antibacterial properties to adhesives, particularly against S. mutans and S. sanguinis. Silver nanoparticles (AgNPs) contribute to increase compressive and tensile strength [74]. Also they provide strong antibacterial effect while maintaining integrity of the adhesive layer [75]. Degrazia et al, [76] and Eslamian et al, [77] reported that silver nanoparticles incorporated into orthodontic adhesives have shown significant antibacterial effects against cariogenic bacteria, particularly Streptococcus mutans. Silica-doped nanohydroxyapatite (Si-NHAP) contributes to improved bond stability and remineralization, helping to form a hydrolytically resistant hybrid layer that sustains high bond strength over time [78].
Zirconia nanoparticles further enhance flexural strength and resistance to degradation [79]. Kumar et al, [80] and Lohbauer et al, [81] Zirconia nanoparticles significantly improved bond strength to dentin when added to commercial adhesive systems. Calcium phosphate nanoparticles promote remineralization, therby enhancing both mechanical properties and the longevity of the adhesive bond [82]. Fluorinated nanoparticles reinforce the adhesive structure and provide the added benefit of fluoride release, which provides additional enamel protection [83]. Zinc oxide nanoparticles (ZnO NPs) exhibit both antimicrobial and remineralization effects, strengthening hybrid layer and supporting the durability of dental restorations. Saffarpour et al, [84] have demonstrated that ZnO NPs can enhance the antibacterial properties of dental adhesives and composites without compromising bond strength. Osorio et al, [85] and Toledano et al, [86] reported that These nanoparticles also promote dentin remineralization, improving the quality of resin-dentin interfaces and increasing long-term bond durability. Finally, graphene-oxide is a promising nanomaterial additive that improves both the mechanical and biological properties of universal dental adhesives, supporting enhanced bond strength, antibacterial protection and restoration longevity [11].
4.1. Chitosan
Chitosan is a naturally derived marine polymer with linear amino-polysaccharide structure that is is widely used in dentistry. It is obtained by deacetylation of the chitin found in exoskeletons of crustaceans (also known as shellfish scaffolds). It also occurs naturally in mammalian cells. Chitosan is valued for its biocompatibility, non-toxicity, regenerative properties, natural availability and its capacity for chemical modification [9].
The potential of chitosan in dentistry was first explored in the early 2000s, focusing on its antimicrobial and biocompatible properties. Researchers examined its ability to inhibit oral bacteria, particularly Streptococcus mutans, which is implicated in dental caries. Studies demonstrated that shellfish scaffold could be incorporated into dental materials without compromising their mechanical properties. Moreover, research explored its potential in dentin remineralization by facilitating calcium and phosphate ion deposition, suggesting a role in enhancing enamel and dentin repair [87].
4.1.1. Properties of chitosan
Incorporation of this cationic polysaccharide into adhesive resins has demonstrated significant inhibitory effects on the growth of Streptococcus mutans, a primary bacterium associated with dental caries. Experimental adhesives containing chitosan at concentrations ranging from0.12% to 1% (w/w) showed that even the lowest concentration effectively reduced bacterial proliferation without compromising adhesive properties. The antibacterial activity was confirmed both in freshly prepared and aged adhesives, indicating chitosan's sustained antimicrobial effect compared to unmodified adhesives [87,88].
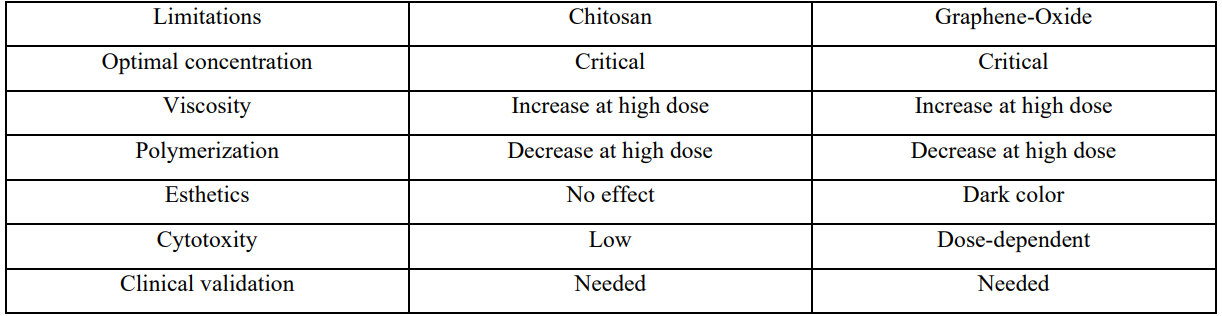
Mechanically, the inclusion of chitosan influences adhesive properties. Increasing chitosan concentration leads to higher viscosity and a decrease in the degree of conversion and pH of the adhesive resin. Adhesives with 0.12% and 0.25% deacetylated chitin maintained microtensile bond strength comparable to controls, whereas higher concentrations (0.5% and 1%) significantly reduced bond strength, highlighting the importance of optimizing chitosan concentration to preserve adhesive performance [87, 88].
Regarding biocompatibility, this marine-derived biopolymer is well-recognized for being biocompatible and biodegradable, making it suitable for intraoral applications [89]. Its bio-adhesive properties allow it to bind effectively to negatively charged surfaces like mucosal membranes, enhancing drug transport across epithelial barriers and supporting its use in dental materials. When incorporated in etch-and-rinse adhesive systems, shellfish-derived polymer has been shown to improve bond strength and preserve the longevity of the resin-dentin interface. This enhancement is partly due to chitosan's ability to inhibit collagen degradation and reduce water permeability, leading to a more stable hybrid layer [90].
4.1.2. Effect of chitosan on bond durability
Diolosà et al, [91] reported that chitosan treatment helped preserve bond strength and reduced collagen breakdown, while Paschoini et al, [90] showed that dentin pretreatment with chitosan improved both immediate and long-term bond strength when combined with either etch-and-rinse or self-etch adhesive systems. Chitosan has been shown to reduce nanoleakage and maintain microtensile bond strength after extended water storage periods of 12 and 24 months, primarily due to its ability to increase the stiffness of demineralized dentin and inhibit matrix metalloproteinases (MMPs) that degrade collagen within the hybrid layer [92]. By forming inter- and intramolecular cross-links with dentinal collagen, chitosan reinforces and stabilizes the collagen fibrils, making them more resistant to enzymatic breakdown and hydrolytic degradation. This cross-linking effect decreases the release of hydroxyproline, a marker of collagen degradation, and leads to a more stable hybrid layer with less nanoleakage over time [92].
Additionally, this naturally sourced amino polysaccharide enhances remineralization by promoting calcium and phosphate ion deposition in dentin, which further enhances the resistance of the dentin surface to MMP activity. The high alkalinity of these nanoparticles (pH ~11) may also contribute to inhibiting MMP activation, which is typically stimulated in acidic environments during bonding procedures. Moreover, the hydrophilic and positively charged characteristics of this marine biopolymer improves dentin surface wettability, facilitating better adhesive infiltration and stronger chemical interactions between the adhesive, chitosan, and dentin collagen. Together, these mechanisms explain why chitosan treatment before adhesive application improves bond durability by preserving the integrity of the resin-dentin interface and reducing collagen degradation over time.[8,93].
Zidan [94] found that pretreatment with this natural polysaccharide before adhesive application improved resin-dentin bond durability over 24 months. Paschoini et al, [95] concluded that incorporating chitosan into adhesive systems enhanced immediate bond strength and preserved it after 6 months of aging. Diolosà et al, [96] reported that a methacrylate-modified chitosan maintained bond strength after thermo-mechanical cycling. Baena et al, [8] reported that application of shellfish-derived polymer led to reduced MMPs activity in dentin. However, Baena et al, [8] and Rafael et al, [97] concluded that no significant effect of chitosan addition on immediate bond strength.
4.1.3. Antibacterial properties of chitosan
This marine-derived biopolymer improves the antibacterial properties of dental adhesives primarily due to its cationic nature, which disrupts bacterial cell membranes and inhibits the growth of oral pathogens such as Streptococcus mutans. This inherent antimicrobial action makes the compound an effective agent in reducing bacterial colonization on adhesive surfaces [98,99]. Additionally, the antibacterial activity of modified adhesives is influenced by pH, with enhanced effectiveness observed in slightly acidic conditions that commonly occur in the oral environment [99]. Recent advances have also explored the use of nanoparticles derived from the polysacharide, which can be combined with other antimicrobial agents like grape seed extract, significantly increasing bacterial inhibition and offering a promising strategy to boost the antibacterial efficacy of dental adhesives. These mechanisms collectively contribute to the improved antimicrobial performance of chitosan-containing dental adhesives without severely compromising their adhesive properties [100].
Several studies [88,98,101,102] have investigated the incorporation of biopolymer into dental adhesives to enhance antibacterial properties. Modified adhesives demonstrated inhibitory effects against Streptococcus mutans. Elsaka et al, [58] and El Azzazy et al, [87,99] concluded that the antibacterial activity increased with higher chitosan concentrations, but excessive amounts negatively impacted adhesive properties. Methacrylate form of the polymer and triclosan-loaded chitosan also showed promising results in reducing biofilm formation [103,104]. Marrwa et al, [105] found that its inclusion in glass ionomer cements improved antibacterial properties without compromising dentin bonding at low concentrations. However, Shahid et al, [106] reported no significant antibacterial effects with certain modifications of the compound.
4.1.4. Effect of chitosan on shear bond strength (SBS)
Mohammed et al, [107] investigated the effects of adding chitosan nanoparticles (CS-NPs) at concentrations of 1%, 5%, and 10% to Transbond™ XT primer. The results indicated no statistically significant differences in SBS among the groups, suggesting that incorporating CS-NPs up to 10% does not adversely affect the bond strength of the adhesive. Abdul-Razzaq et al, [108] examined the effect of dentin surface pretreatment with a 0.2% polysaccharide solution on the SBS of an etch-and-rinse adhesive system. The findings revealed that such pretreatment did not significantly influence the SBS, indicating that chitosan application at this concentration is compatible with the adhesive system used. Perchyonok et al, [109] assessing the impact of chitosan hydrogels on dentin bond strength observed an initial improvement in SBS after 24 hours. However, a decrease in bond strength was noted after six months, highlighting the need for further research to enhance the long-term stability of such adhesive modifications.
4.1.5. Effect of chitosan on curing time
Degree of conversion reflects the extent to which monomers in a resin-based material are polymerized into a solid network, influencing mechanical properties and durability. Alkhudhairy et al, [110] investigated the impact of incorporating 1% chitosan into an adhesive system and reported no significant change in the DC compared to the control group. However, this incorporation led to an improvement in SBS, suggesting that chitosan enhances mechanical properties without adversely affecting the polymerization efficiency of the photoinitiator system.
The interaction of chitosan with various photoinitiators has been explored to assess its influence on the curing process and resultant material properties. Silva et al, [111] demonstrated that addition of biopolymer to experimental resins containing camphorquinone (CQ) as the photo initiator resulted in increased mechanical properties, such as flexural strength, without compromising the polymerization process. Moreover, resins with 1% polysaccharide exhibited enhanced human keratinocyte viability, indicating improved biocompatibility alongside mechanical reinforcement.
4.1.6. Solvent role in addition of chitosan to dental adhesives
Solvent choice is critical for dissolving the cationic biopolymer, ensuring chemical interaction and functional integration. Its bio-adhesive properties rely on electrostatic interactions between its amine groups and dentin collagen, facilitated by solvents that maintain molecular structure while enabling cross-linking with the dental substrate [112]. The polysaccharide is typically insoluble in water but soluble in acidic solutions. Common solvents used for dissolving chitosan include acetic acid, which helps to protonate the amino groups, making it soluble and facilitating its incorporation into dental adhesives [87,90]. The choice of solvent affects the concentration and viscosity of the chitosan solution. For instance, using a 1% acetic acid solution is common for preparing chitosan solutions, which helps maintain a consistent viscosity that is compatible with dental adhesives [87]. Bettiol et al, [113] using 1% chitosan in universal adhesive systems (e.g., Single Bond Universal) demonstrate that solvents must balance chitosan dispersion without altering the adhesive’s colloidal stability or polymerization efficiency, as improper solvent selection could lead to particle agglomeration or reduced interfacial penetration. The solvent must be compatible with other components of the dental adhesive to avoid adverse interactions that could compromise the adhesive's properties. So Optimal solvent systems enable chitosan to enhance mechanical properties like bond strength and flexural resistance without compromising dentin permeability or adhesive layer micromorphology [112,113].
4.1.7. Limitations of addition chitosan to dental adhesive
While the additive offers antibacterial benefits, its inclusion lowers the pH of the adhesive system, increasing its acidity and potentially affecting the adhesive's performance and compatibility with dental tissues [99]. Azzazy et al, [88] found that chitosan incorporation reduce pH from around 5.55 to 4.71 due to acetylation in acetic acid during preparation. Higher concentrations also increase the viscosity of the adhesive, potentially hindering its handling and application. Moreover, elevated chitosan levels decrease the degree of conversion of adhesive monomers, negatively impacting mechanical properties and durability of the adhesive. This is accompanied by a significant reduction in bond strength to dentin at concentrations of 0.5% and 1% [87]. Although polymer can cross-link with collagen, potentially enhancing hybrid layer stability, its role in improving long-term bond durability remains inconclusive. Baena et al, [8] showed that it does not significantly enhance bond longevity when used as a primer. Despite, promising in vitro findings, clinical evidence supporting the real-world effectiveness of chitosan-modified adhesives remains limited, underscoring the need for rigorous clinical trials to validate long-term performance [114].
4.2. Graphene-oxide
Incorporating this carbon-based nanomaterial into dental adhesives has garnered significant attention due to its unique structural properties that enhance adhesive performance. It is composed of a single-layered carbon sheet functionalized with oxygen-containing groups such as hydroxyl, epoxy and carboxyl groups on its basal plane and edges. These functional groups facilitates strong interactions with resin monomers and dental substrates, leading to improved mechanical properties and increased bond strength [115].
4.2.1. Properties of graphene-oxide
Integration of the oxidized graphene derivative into dental adhesives offers multiple beneficial properties that enhance their overall performance. Mechanically, the addition of this two-dimensional nanomaterial has been shown to improve SBS of adhesives, contributing to more durable dental restorations. Rygas al et, [11] revealed that inclusion of this sheet-like additive enhances SBS and reduces the Adhesive Remnant Index (ARI), indicating superior adhesive performance. Importantly, these improvements were achieved without negatively impacting the biocompatibility of the adhesives, ensuring their safety for oral tissues. Additionally, graphene-oxide enhances the water sorption and solubility of dental adhesives; Pereira et al, [116] concluded that its hydrophilic nature helps decrease these parameters, which is crucial for maintaining the structural integrity and longevity of restorations.
Beyond mechanical improvements, Williams et al, [117] demonstrated that carbonaceous additive supports cell adhesion, proliferation, and differentiation without inducing cytotoxic effects on oral cells such as fibroblasts, making it a safe choice for dental applications. Furthermore, the material exhibits strong antibacterial and antibiofilm activities, effectively suppressing
Streptococcus mutans-the primary bacterium responsible for dental caries-and inhibiting bacterial colony formation on tooth surfaces. These properties help reduce the risk of secondary caries and other oral diseases, positioning nanomaterial as a highly promising material for enhancing the functionality and longevity of dental adhesives [117,118].
4.2.2. Effect of graphene-oxide on bond durability
Bin-shuwaish et al, [119] and AlFawaz et al, [120] concluded that incorporating this functionalized carbon sheet improves microtensile bond strength and durability of adhesives to dentin [121,122]. While Gamal et al, [123] reported no significant impact on shear bond strength. Basil et al, [124] found increased bond strength with graphene-oxide incorporation. Liu et al, [125] showed adhesives contain oxidized graphene improved degree of conversion. However, Bin-Shuwaish et al, [121] found that concentrations may decrease the degree of conversion. Overall, incorporation of this advanced nanomaterial in dental adhesives appears promising for enhancing bond strength and durability, though optimal concentrations and combinations with other nanofillers require further investigation [126,127].
4.2.3. Antibacterial properties of graphene-oxide
The oxidized graphene derivative enhances the antibacterial properties of dental adhesives through multiple complementary mechanisms, making it as a highly effective additive for combating oral pathogens and preventing secondary caries. One primary mode of action is physical membrane disruption: Nano-sharp edges of graphene-oxide mechanically puncture bacterial cell walls such as those of Streptococcus mutans and Porphyromonas gingivalis leading to cytoplasmic leakage and cell death. This mechanical damage is effective against Gram-positive and Gram-negative bacteria [117,118]. Additionally, nanosheets can wrap around bacterial cells, effectively isolating them from nutrients and inhibiting their proliferation [11,117]. Bregnocchi et al, [118] demonstrated that dental adhesives containing 2D carbon material exhibit significant anti-bacterial and antibiofilm activities, effectively suppressing Streptococcus mutans without compromising bonding efficiency.
Beyond physical damage, this nanofiller induces oxidative stress by generating reactive oxygen species (ROS) that degrade bacterial lipids, proteins, and DNA, disrupting metabolic functions and preventing biofilm formation [11,117]. Moreover, synergistic antibacterial effects are observed when material is combined with metal nanoparticles like silver. Silver-doped graphene-oxide nanocomposites amplify ROS production and interfere with bacterial glycolysis, achieving bacterial inhibition rates up to 60% in dental adhesives [117,118]. These multifaceted antibacterial mechanisms make advanced carbon nanomaterial a promising material for enhancing the antimicrobial efficacy of dental adhesives.
4.2.4. Effect of graphene-oxide on shear bond strength
This two-dimensional nanofiller enhances the bond durability of dental adhesives through multiple synergistic mechanisms that collectively improve mechanical performance and interfacial stability. Its exceptional mechanical strength and stiffness reinforce the adhesive matrix, increasing its resistance to mechanical stress and long-term degradation [12]. This reinforcement is further amplified when combined with other nanofillers, such as calcium phosphate (CaP), creating synergistic interactions that optimize bond strength; Alomran et al, [128] found that composite containg 5 wt% of carbon-based additives and CaP demonstrated superior bond strength compared to conventional adhesives. Additionally, adhesives modified with this nanomaterial reduce nanoleakage-a critical factor in bond degradation-by forming a more homogeneous adhesive interface that minimizes microgaps and prevents hydrolytic breakdown. Studies show that in the absence of nanoleakage, adhesives containing graphene derivative achieve bond durability comparable to traditional systems while maintaining structural integrity under cyclic mechanical and thermal stresses [12,115]. These combined effects position oxidized carbon sheet as a transformative additive for improving the longevity of dental restorations.
Ghodrati et al, [10] evaluated the effect of adding nano-formulation of material to conventional glass ionomer cement (CGIC) and resin-modified glass ionomer cement (RMGIC). The research demonstrated that incorporating 1 wt.% and 2 wt.% nanofiller significantly increased the SBS to dentin in both groups, suggesting improved adhesive performance.
Gamal et al, [123] investigated the incorporation of nanoparticles of this carbon material into a self-etch adhesive system, assessing both antibacterial properties and SBS after adding 2% and 5%. While a dose-dependent antibacterial effect was observed, the addition did not significantly affect the SBS of the adhesive, indicating that nanomaterial can impart antibacterial properties without compromising the bonding efficacy of self-etch adhesives.
Liu et al, [129] found that incorporating graphene increased the SBS of both adhesive, with 0.01 and 0.05 wt.% addition resulting in a statistically significant enhancement in SBS, suggesting that even low concentrations can positively influence the bonding performance of orthodontic adhesives. Alrafee et al, [123] explored the effects of incorporating oxidized carbon nanomaterial into self-etch adhesives on both antibacterial properties and SBS. The findings indicated that while additive provided a significant antibacterial effect, it did not adversely affect the SBS of the adhesive, highlighting the potential to enhance the functional properties of dental adhesives without compromising their mechanical performance.
4.2.5. Effect of graphene-oxide on curing time
Degree of conversion which indicates the extent of monomer polymerization in dental adhesives and directly impacts their mechanical properties and durability, can be affected by the incorporation of this carbon-based nanomaterial. Bin-Shuwaish et al, [119] investigated adhesives containing 0.5% and 2.0% nanosheets by weight and found that while the control adhesive exhibited a DC of 46.8%, addition of 0.5% and 2.0% reduced the DC to 42.3% and 37.7%, respectively. This reduction suggests that higher nanomaterial concentrations may interfere with the polymerization process, possibly due to interactions with photoinitiator system. Correspondingly, Martini et al, [12] observed that the additive influences the mechanical properties of dental adhesives after photo-curing, likely linked to its interaction with the photoinitiator during polymerization. These findings highlight need to optimize nanofiller concentration to avoid compromising polymerization efficiency and mechanical performance.
4.2.6. Solvent role in addition of graphene-oxide to dental adhesives
The role of the solvent is critical for nanosheet dispersion, interfacial bonding, and structural integrity of the adhesive system. The polarity of the solvent affects oxidized graphene configuration: in aqueous solvents, the sheets tend to crumple due to hydrogen bonding and capillary forces during drying, reducing surface area and mechanical reinforcement potential [130]. In contrast, solvents like acetone facilitate better dispersion and covalent bonding with polymer chains, especially when adding early in the synthesis process, as seen in polyurethane-urea adhesives [131]. Hydrophilic solvents such as HEMA, commonly used in dentin adhesives, improve nanomaterial dispersion by aligning with its hydrophilic nature; however, excessive water absorption can lower adhesive viscosity, limiting penetration of graphene-oxide into the hybrid layer and dentin tubules [12].
Chemical interactions between 2D material and the adhesive matrix also depend heavily on the solvent environment. For example, adding oxidized graphene during prepolymer formation in acetone promotes covalent bonding with isocyanate groups, enhancing mechanical properties like T-peel strength. When introduced later, nanosheets act as a physical filler, improving lap-shear strength but potentially reducing peel strength [131]. In dental adhesives using ethanol-water solvent mixtures, nanofiller form hydrogen bonds with collagen fibrils, reinforcing micromechanical interlocking within the hybrid layer; however, excessive hydrophilicity may accelerate nanoleakage and compromise bond durability over time [12]. Functionally, optimal solvent selection ensures sheet-like structures remain flat and well-dispersed, maximizing their surface area for interaction. For instance, incorporating 1–2 wt.% additive in (RMGIC) improves shear bond strength due to enhanced hybrid layer penetration [10]. Additionally, solvents that preserve sheet-like structure , such as acetone, enhance its mechanical reinforcement effects, while aqueous environments may reduce structural stability but maintain antibacterial benefits [130].
4.2.7. Limitations of addition graphene-oxide to dental adhesive
While nanomaterial enhances mechanical strength and antibacterial activity, limitation exist. Its inherent dark coloration compromise the esthetics of dental restorations, particularly in anterior teeth where appearance is critical [116]. Cytotoxicity is concentration-dependent: Rygas et al, [11] concluded that low concentrations (e.g., 0.25 wt%) show minimal cytotoxic effects with cell survival rates above 80%, higher concentrations (e.g., 0.5 wt%) have demonstrated significant cytotoxicity after 48 hours, underscoring the need for careful optimization of graphene-oxide content. Achieving uniform dispersion is challenging due to nanosheet agglomeration leading to inconsistencies properties [12].
Additionally the additive alters the viscosity, complicating clinical application and possibly requiring adjustments in technique to maintain optimal performance [132].
Moreover, the long-term stability under oral conditions remain uncertain. Factors such as hydrolytic degradation, wear resistance, and the adhesive’s behavior under cyclic mechanical loading have yet to be thoroughly evaluated [132]. Addressing these limitations through further research and formulation refinement is essential to fully realize benefits of nanosheet.
Conclusion
Incorporation of bioactive and nanostructured additives such as chitosan and graphene-oxide have markedly enhanced the performance and longevity of bonded restorations. These innovations directly address longstanding challenges in dentin bonding, including hydrolytic and enzymatic degradation and bacterial infiltration, which are primary contributors to restoration failure and secondary caries. Chitosan has demonstrated the ability to stabilize the dentin matrix, inhibit matrix metalloproteinases, and provide sustained antimicrobial effects, all while promoting remineralization and maintaining biocompatibility. Graphene-oxide, with its unique nanostructure, not only reinforces mechanical properties and bond durability but also imparts potent antibacterial activity and reduces nanoleakage at the adhesive interface.
Despite these promising developments, several limitations persist. The optimal concentrations of these additives must be carefully balanced to avoid adverse effects on adhesive viscosity, polymerization, and esthetics—especially in visible restorations. Furthermore, while in vitro and short-term studies support the efficacy of these advanced adhesives, there remains a need for robust clinical trials to confirm their long-term benefits and real-world applicability. Continued research and formulation refinement are essential to fully realize the potential of antibacterial dental adhesives and to establish their role in extending the clinical lifespan of restorative treatments.
Summary Visuals
1. Key Challenges in Dental Adhesives

2. Effects of Chitosan and Graphene-Oxide Additives

3. Mechanisms of Action
4. Limitations and Future Directions
References
- B Van Meerbeek., K Yoshihara., K Van Landuyt., Y Yoshida., M Peumans. From Buonocore's Pioneering Acid–Etch Technique to Self–Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J Adhes Dent. 2020;22(1):7–34. [PubMed.]
- D M J Van Meerbeek B., Yoshida., et al. Adhesion to enamel and dentin:current status and future challenges. J Dent Res. 2015;82(3):204–217. [Ref.]
- F J Braga RR. Resin–based composite restorations a review of the current dental adhesive systems and their effectiveness. J Prosthet Dent. 2015;94(1):1–13. [Ref.]
- D E Betancourt., P A Baldion., J E Castellanos. Resin–Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer, International. Journal of Biomaterials. 2019;1–11. [PubMed.]
- A Frassetto., L Breschi., G Turco., G Marchesi., R Di Lenarda. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability. A literature review, Dent Mater. 2016;32(2):41–53. [PubMed.]
- L Tjäderhane., F D Nascimento., L Breschi., A Mazzoni. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013;29(1):116–35. [PubMed.]
- L S Mokeem., I M Garcia., M A Melo. Degradation and Failure Phenomena at the Dentin Bonding Interface. Biomedicines. 2023;11(5). [PubMed.]
- E Baena., S R Cunha., T Maravić,. A Comba., F Paganelli., et al. Effect of Chitosan as a Cross–Linker on Matrix Metalloproteinase Activ ity and Bond Stability with Different Adhesive Systems. Marine Drugs. 2020;18(5):263. [PubMed.]
- A Paradowska–Stolarz., M Mikulewicz., J Laskowskam., B Karolewicz., A Owczarek. The Importance of Chitosan Coatings in Dentistry. Marine Drugs. 2023;21(12):613. [PubMed.]
- P Ghodrati., F Sharafeddin. Evaluation of the effect of nano–graphene oxide on shear bond strength of conventional and resin–modified glass ionomer cement. Clin Exp Dent Res. 2023;9(5):851–858. [PubMed.]
- J Rygas., J Matys., M Wawrzyńska., M Szymonowicz., M Dobrzyński. The Use of Graphene Oxide in Orthodontics–A Systematic Review. J Funct Biomater. 2023;14(10). [PubMed.]
- A Alshahrani., M S Bin–Shuwaish., R S Al–Hamdan., T Almohareb., et al . Graphene oxide nano–filler based experimental dentine adhesive. A SEM / EDX, Micro–Raman and microtensile bond strength analysis. Journal of Applied Biomaterials & Functional Materials. 2020;18:2280800020966936. [PubMed.]
- W Channarong., N Lohawiboonkij., P Jaleyasuthumkul., K Ketpan., N Duangrattanaprathip., K Wayakanon. Fracture resistance of bonded ceramic overlay restorations prepared in various designs. Scientific Reports. 2022;12(1):16599. [PubMed.]
- A Tsujimoto., W W Barkmeier., N G Fischer., K Nojiri., Y Nagura., et al. Wear of resin composites: Current insights into underlying mechanisms, evaluation methods and influential factors. Jpn Dent Sci Rev. 2017;54(2):76–87. [Ref] [PubMed.]
- D Mario., A Mario., C Allegra., B Andrea., T Giuseppe., et al. The influence of indirect bonded restorations on clinical prognosis of endodontically treated teeth: A systematic review and meta–analysis. Dent Mater. 2022;38(8):e203–e219. [PubMed.]
- P Monga., V Sharma., S Kumar. Comparison of fracture resistance of endodontically treated teeth using different coronal restorative materials: An in vitro study. Journal of conservative dentistry :JCD. 2009;12(4):154–159. [PubMed.]
- B Sagsen., B Aslan. Effect of bonded restorations on the fracture resistance of root filled teeth. Int Endod J. 2006;39(11):900–4. [PubMed.]
- C Tennert., C Maliakal., L Suarèz Machado., T Jaeggi., H Meyer–Lueckel., J Wierichs Richard. Longevity of posterior direct versus indirect composite restorations: A systematic review and meta–analysis. Dental materials : official publication of the Academy of Dental Materials. 2024;40(11):e95–e101. \ [PubMed.]
- K Bhargava., C Mastud., S K Mastud., D M Vikhe., P Newase., P N Mhaske. Recent Advances in Direct Reinforced Restorations for Vital Teeth, Advances in Human Biology. 2022;12(2). [Ref.]
- MB Blatz. Adhesive Dentistry: Just Bond It!. Compend Contin Educ Dent. 2021;42(9):536–537. [PubMed.]
- D A N A., Cardoso MV., Mine A., Coutinho E., et al. Current aspects on bonding effectiveness and stability in adhesive dentistry. Australian Dental Journal. 2011;56:31–44. [PubMed.]
- Stape T H S., Cibelik HS., Tjäderhane L., Tezvergil–Mutluay A. Dry bonding to dentin: broadening the moisture spectrum and increasing wettability of etch–and–rinse adhesives. Dental Materials. 2021;37:1676–1687. [PubMed.]
- M A Breschi L., Nato F., Carrilho M., Tjäderhane L., Ruggeri A., et al. Current trends in dentin bonding: From macro to nano, Dental Materials. 2018;34(5):637–55. [Ref.]
- M A Maravic T., Comba A., Checchi V., Scotti N., Breschi L. How stable is dentin as a substrate for bonding?, Current Oral Health Reports. 2019;6(3):179–87. [PubMed.]
- J Fan., P Wang., S Wang., R Li., Y Yang., et al. Advances in macro–bioactive materials enhancing dentin bonding. Discov Nano. 2025;20(1):40. [PubMed.]
- K H Alshaikh., H H H Hamama., S H Mahmoud. Effect of smear layer deproteinization on bonding of self–etch adhesives to dentin: a systematic review and meta–analysis. Restor Dent Endod. 2018;43(2):e14. [PubMed.]
- P Saikaew., V Sattabanasuk., C Harnirattisai., A Chowdhury., R Carvalho., H Sano. Role of the smear layer in adhesive dentistry and the clinical applications to improve bonding performance. Jpn Dent Sci Rev. 2022;58;59–66. [PubMed.]
- D R W Cuevas–Suárez CE., Lund RG., Stanislawczuk R., Loguercio AD., Piva E. Dentin bonding: State of the art and future challenges. Dental Materials Journal. 2019;38(5):703–9. [Ref.]
- Yoshihara K., Nagaoka N., Okihara T., Kameyama A., Maruo Y., et al. Etching efficacy of self–etching primers on ground and unground enamel. Journal of Dentistry 2020;95:103298. [Ref.]
- M Souza., L Meirelles., I Duque., M Mailart., T Caneppele., E Bresciani., Determination of chemical elements and cross–section hardness of sclerotic darkened human dentin. Brazilian Dental Science. 2017;20. [Ref.]
- C Chen., L N Niu., H Xie., Z Y Zhang., L Q Zhou., et al. Bonding of universal adhesives to dentine–Old wine in new bottles. J Dent. 2015;43(5):525–36. [PubMed] [PubMed.]
- M M Stape THS., Tjäderhane L., Uurasjärvi E., Koistinen A., Tezvergil–Mutluay A. The pursuit of resin–dentin bond durability: simultaneous enhancement of collagen structure and polymer network formation in hybrid layers. Dental Materials. 2021;37:1083–1095. [PubMed.]
- L Tjäderhane. Dentin Bonding: Can We Make it Last?. Operative Dentistry. 2015;40(1):4–18. [PubMed.]
- W K Alomran., M Z I Nizami., H H K Xu., J Sun. Evolution of Dental Resin Adhesives–A Comprehensive Review. J Funct Biomater. 2025;16(3). [PubMed.]
- L Breschi., T Maravic., S R Cunha., A Comba., M Cadenaro., et al. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent Mater. 2018;34(1):78–96. [PubMed] [PubMed.]
- Y Liu., L Tjäderhane., L Breschi., A Mazzoni., N Li., et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90(8):953–68. [PubMed.]
- C Sabatini., D H Pashley. Mechanisms regulating the degradation of dentin matrices by endogenous dentin proteases and their role in dental adhesion. A review. Am J Dent. 2014;27(4):203–14. [PubMed.]
- N d O Sousa., S J M Maia., K E R Lima., M M Moreira., T O Rifane., et al. Incorporation of epigallocatechin–3–gallate absorbed onto hydroxyapatite nanoparticles in dental adhesive: Impact on resin dentin–bonding, chemical, and mechanical properties. International Journal of Adhesion and Adhesives. 2025;140:104019. [Ref.]
- I Q S de Moraes., T G do Nascimento., A T da Silva., L de Lira., I Porto. Inhibition of matrix metalloproteinases: a troubleshooting for dentin adhesion. Restor Dent Endod. 2020;45(3):e31. [PubMed.]
- B L Zarella., C A Cardoso., V T Pelá., M T Kato., L Tjäderhane., M A Buzalaf. The role of matrix metalloproteinases and cysteine–cathepsins on the progression of dentine erosion. Arch Oral Biol. 2015;60(9):1340–5. [PubMed.]
- R B Yaghmoor., H Jamal., H Abed., E Allan., P Ashley., A Young. Incorporation of MMP inhibitors into dental adhesive systems and bond strength of coronal composite restorations: A systematic review and meta–analysis of in vitro studies. Jpn Dent Sci Rev. 2022;58:298–315. [PubMed.]
- L Breschi., P Martin., A Mazzoni., F Nato., M Carrilho., L Tjäderhane., et al. Use of a specific MMP–inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010;26(6):571–8. [PubMed.]
- A Albaladejo., R Osorio., M Toledano., M Ferrari. Hybrid layers of etch–and–rinse versus self–etching adhesive systems. Med Oral Patol Oral Cir Bucal. 2010;15(1):e112–8. [PubMed.]
- X V Tran., K Q Tran. Microleakage and characteristics of resin–tooth tissues interface of a self–etch and an etch–and–rinse adhesive systems. Restor Dent Endod. 2021;46(2):e30. [PubMed] [PubMed.]
- P R Mohanty., L Mishra., K Saczuk., B Lapinska. Optimizing Adhesive Bonding to Caries Affected Dentin: A Comprehensive Systematic Review and Meta–Analysis of Dental Adhesive Strategies following Chemo–Mechanical Caries Removal, Applied Sciences. 2023;13(12). [PubMed.]
- U M Stape THS., Cibelik HS., Tjäderhane L., Tezvergil–Mutluay A. Dry bonding to dentin: broadening the moisture spectrum and increasing wettability of etch–and–rinse adhesives. Dent Mater. 2021;37(11):1676–1687. [PubMed.]
- T F Pashley DH., Breschi L., et al. Enzyme inhibitors in bonding to caries–affected dentin: A review. J Dent Res. 2021;100(6):529–536. [Ref.]
- C Müller., G Rosa., G Teixeira., I Krejci., T Bortolotto., H Alexandre. Susin, Effect of caries–affected dentin on one–step universal and multi–step etch–and–rinse adhesives’ bond strength. Rev Odontol UNESP. 2017;46. [Ref.]
- R Pavanello., S L Pinheiro. Influence of Phosphoric Acid Etching on the Bond Strength of a Universal Adhesive System to Caries–Affected Dentin. Advances in Biological Chemistry. 2018;08(03):37–46. [PubMed.]
- CP Isolan., R Sarkis–Onofre., G S Lima., R R Moraes. Bonding to Sound and Caries–Affected Dentin: A Systematic Review and Meta–Analysis. J Adhes Dent. 2018;20(1):7–18. [PubMed] [PubMed.]
- G F Nicoloso., B F Antoniazzi., T L Lenzi., F Z Soares., R O Rocha. Is There a Best Protocol to Optimize Bond Strength of a Universal Adhesive to Artificially Induced Caries–affected Primary or Permanent Dentin?. J Adhes Dent. 2016;18(5):441–446. [PubMed.]
- Z Al–Obaidi., H Jasim. Assessment of Shear Bond Strength to Sound and Artificial Caries Affected Dentin Using Different Adhesive Systems. An In Vitro Study. Dental Hypotheses. 2023;14:10. [Ref.]
- P J. Current perspectives on dental adhesion. Journal of Adhesive Dentistry. 2017;19(2):7–20. [Ref.]
- VMB Yoshida Y. Bonding effectiveness of adhesive monomers. Dental Materials. 2018;34(3):345–356. [Ref.]
- E A Hanabusa M. Effects of solvent content in universal adhesives. Journal of Dental Research. 2019;98(1):67–75. [Ref.]
- A Ghyadh., H Ragab., E Osman. Shear Bond Strength of a Multi–Mode Universal Adhesive Containing Hydroxy–Apatite Nanoparticles to Dentin (In vitro study), Egyptian. Dental Journal. 2021;67:2749–2758. [Ref.]
- E Sofan., A Sofan., G Palaia., G Tenore., U Romeo., G Migliau. Classification review of dental adhesive systems: from the IV generation to the universal type, Ann Stomatol (Roma). 2017;8(1):1–17. [PubMed.]
- E Van Landuyt KL. Evaluation of self–etch adhesive longevity, Clinical Oral Investigations. 2020;24(5):1783–1792. [Ref.]
- A A Elkaffas., H H H Hamama., S H Mahmoud. Do universal adhesives promote bonding to dentin. A systematic review and meta–analysis. Restor Dent Endod. 2018;43(3):e29. [PubMed.]
- S Nagarkar., N Theis–Mahon., J Perdigão. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J Biomed Mater Res B Appl Biomater. 2019;107(6):2121–2131. [PubMed.]
- J Perdigão., A D Loguercio. Universal or Multi–mode Adhesives: Why and How?. J Adhes Dent. 2014;16(2):193–4. [PubMed.]
- L Breschi. Buonocore Memorial Lecture 2023: Changing Operative Mindsets with Universal Adhesives and Cements. Oper Dent. 2025;50(1):12–32. [PubMed.]
- W L Rosa., E Piva., A F Silva. Bond strength of universal adhesives: A systematic review and meta–analysis. J Dent. 2015;43(7):765–76. [PubMed.]
- M Sebold., A C Bosso., S B Ometto., B Lorenzo., and M Giannini. Chronological history and current advancements of dental adhesive systems development: a narrative review. Journal of Adhesion Science and Technology. 2021;35(18):1941–1967. [Ref.]
- G Alex. Universal adhesives: the next evolution in adhesive dentistry? Compend Contin Educ Dent. 2015;36(1):15–26; quiz 28,40. [PubMed.]
- G S Carrilho MR., Tay F., et al. Inhibition of MMPs by chlorhexidine. J Dent Res. 2016;95(5):519–525. [Ref.]
- N Kharouf., A Eid., L Hardan., R Bourgi., Y Arntz., et al. Antibacterial and Bonding Properties of Universal Adhesive. Dental Polymers Doped with Pyrogallol, Polymers. 2021;13(10):1538. [PubMed.]
- E Ferrando–Magraner., V García–Sanz., C Bellot–Arcís., A Marín–Gozalbo., L Cabedo–Mas., et al. Improving the Antibacterial Properties of Dental Bonding Materials Loaded with Silver Compounds. Antibiotics (Basel). 2023;12(12). [PubMed.]
- A Mansoor., Z Khurshid., M T Khan., E Mansoor., P J Palma. Medical and Dental Applications of Titania Nanoparticles: An Overview, Nanomaterials (Basel). 2022;12(20). [PubMed.]
- J Sun., A M Forster., P M Johnson., N Eidelman., et al. Improving performance of dental resins by adding titanium dioxide nanoparticles. Dent Mater. 2011;27(10):972–82. [PubMed.]
- R Ali., A Alwan. Titanium Dioxide Nanoparticles in Dentistry: Multifaceted Applications and Innovations, Future. Dental Research. 2023;1. [Ref.]
- A Sodagar., M S A Akhoundi., A Bahador., Y F Jalali., et al. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics, Dental Press. J Orthod. 2017;22(5):67–74. [PubMed.]
- R D Hiers., P Huebner., S S Khajotia., F L E Florez. Characterization of Experimental Nanoparticulated Dental Adhesive Resins with Long–Term. Antibacterial Properties, Nanomaterials (Basel). 2022;12(21). [PubMed] [PubMed.]
- A A Algarni. Antibacterial Agents for Composite Resin Restorative Materials: Current Knowledge and Future Prospects, Cureus. 2024;16(3):e57212. [PubMed.]
- S–V R García–Contreras R., Contreras–Bulnes R., et al. Effects of silver nanoparticles on the antimicrobial and adhesive properties of dental adhesives. Dent Mater J. 2021;40(2):383–90. [Ref.]
- F W Degrazia., V.C Leitune., I M Garcia., R A Arthur., S M Samuel., F M Collares. Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J Appl Oral Sci. 2016;24(4):404–10. [PubMed.]
- L Eslamian., A Borzabadi–Farahani., S Karimi., S Saadat., M R Badiee. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In–Vitro Study. Nanomaterials (Basel). 2020;10(8). [PubMed.]
- P Gonapa., G S Sajjan., A Bhupathi., U K Podugu., S Sundar., et al. Evaluation of Bond Durability, Surface Morphology, and Remineralization at the Adhesive Interface with Dentin Bonding Agents Modified with Silica–doped Nanohydroxyapatite. Contemporary Clinical Dentistry. 2022;13(4). [PubMed.]
- L Q Chen L., Chen S., et al. Reinforcement of dental adhesives with zirconia nanoparticles. Dent Mater. 2019;35(3):e50–e58. [Ref.]
- U Kumar., D Kumar., K N Gosai., D Dalal., B Pragnya., S Nagarajan. Effectiveness of Nanoparticles in Enhancing Bond Strength in Adhesive Dentistry. J Pharm Bioallied Sci. 16(Suppl 4). (2024) S3772–s3774. [PubMed.]
- U Lohbauer., A Wagner,. R Belli., C Stoetzel., et al. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010;6(12):4539–46. [PubMed.]
- W M Melo MAS., Xu HHK. Bioactive calcium phosphate nanoparticles for dental adhesives. J Biomed Mater Res B Appl Biomater. 2022;110(3):531–539. [Ref.]
- L J AF., Garcia FC., et al. Fluorinated dental adhesives: A systematic review. J Adhes Dent. 2023;25(1):67–75. [Ref.]
- M Saffarpour., M Rahmani., M Tahriri., A Peymani. Antimicrobial and bond strength properties of a dental adhesive containing zinc oxide nanoparticles. Brazilian Journal of Oral Sciences. 2016;15(1):66. [Ref.]
- R Osorio., I Cabello., A L Medina–Castillo., E Osorio., M Toledano. Zinc–modified nanopolymers improve the quality of resin–dentin bonded interfaces. Clin Oral Investig. 2016;20(9):2411–2420. [PubMed.]
- M Toledano., M Vallecillo–Rivas., F S Aguilera., M T Osorio., E Osorio., R Osorio. Polymeric zinc–doped nanoparticles for high performance in restorative dentistry. Journal of Dentistry. 107 (2021):103616. [PubMed.]
- S Elsaka. Antibacterial activity and adhesive properties of a chitosan–containing dental adhesive. Quintessence international (Berlin, Germany 1985). 2012;43:603–13. [PubMed.]
- A Azzazy., M Ali., R Abdelrahim. The effect of chitosan incorporation on some properties and antibacterial activity of dental adhesive, Al–Azhar. Journal of Dental Science. 2018;21:357–364. [Ref.]
- A Agrawal., A Reche., S Agrawal., P Paul. Applications of Chitosan Nanoparticles in Dentistry: A Review, Cureus. 2023;15(12):e49934. [PubMed.]
- V L Paschoini., I R Ziotti., C R Neri., S A M Corona., A E Souza–Gabriel. Chitosan improves the durability of resin–dentin interface with etch–and–rinse or self–etch adhesive systems. J Appl Oral Sci. 2021;29:e20210356. [PubMed.]
- M Diolosà., I Donati., G Turco., M Cadenaro., S Paoletti. Use of Methacrylate–Modified Chitosan to Increase the Durability of Dentine Bonding Systems, Biomacromolecules. 2014;15(12):4606–4613. [PubMed.]
- A Z Zidan. Effect of chitosan on resin–dentin interface durability: A 2 year in–vitro study. Egyptian Dental Journal. 2019. [Ref.]
- R Meher., R R Mallick., P Sarangi., A Jena., S Suman., G Sharma. Optimization of chitosan nanoparticle dentin pretreatment with different concentrations and application times to improve bonding at resin–dentin interface. J Conserv Dent Endod. 2025;28(3):248–252. [PubMed] [PubMed.]
- Z A. Effect of chitosan on resin–dentin interface durability: A 2 year in–v itro study. Egyptian dental journal. 2019. [Ref.]
- L Isabella. Chitosan improves the durability of resin–dentin interface with etch–a nd–rinse or self–etch adhesive systems. Journal of Applied Oral Science. 2021. [PubMed.]
- DMDI., et al. Use of methacrylate–modified chitosan to increase the durability of de ntine bonding systems, Biomacromolecules. 2014. [PubMed.]
- A Rafael., et al. Chitosan in different concentrations added to a two–step etch–and–rins e adhesive system: influence on bond strength to dentin. 2017. [Ref.]
- E S. Antibacterial activity and adhesive properties of a chitosan–containin g dental adhesive. Quintessence International. 2012. [PubMed.]
- A M El Azzazy., M S Ali., R A Abdelrahim. The effect of chitosan incorporation on some properties and antibacterial activity of dental adhesive, Al–Azhar. Journal of Dental Science. 2018;21(4):357–364. [PubMed.]
- N Moustafa., I Motawae., R Bayoumi. Evaluating the Antibacterial Activity of Prepared Chitosan Nanoparticles Loaded with Grape Seed Extract Incorporated into Dental Adhesive, Al–Azhar. Journal of Dentistry. 2024. [Ref.]
- L M T., C, A F F., F B R. Chitosan incorporated in a total–etch adhesive system: antimicrobial a ctivity against Streptococcus mutans and Lactobacillus casei. General dentistry. 2017. [PubMed.]
- Y Shun–yi., C Si–peng., W Ruolan., Z Kai., L Xiaoming. Antibacterial activity and bonding performance of carboxymethyl chitos an–containing dental adhesive system, International. Journal of Adhesion and Adhesives. 2022. [Ref.]
- H S M Ana., G I S M A L., V C F. Triclosan–loaded chitosan as antibacterial agent for adhesive resin. E –journal of dentistry. 2019. [PubMed.]
- S R S Ida., R H S., D I K H. Effect of methacrylated chitosan incorporated in experimental composit e and adhesive on mechanical properties and biofilm formation, European. J Oral Sciences. 2019;127(1):81-88. [PubMed.]
- A I Marrwa., N J J E R., F A. Characterization of antibacterial and adhesion properties of chitosan– modified glass ionomer cement. J Biomater Appl. 2015. [PubMed.]
- A Shahid., S Laila., K Naresh., K B K., Z S Z. Muhammad, Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites. THC. 2019. [Ref.]
- R R Mohammed., R A Rafeeq. Evaluation of the Shear Bond Strength of Chitosan Nanoparticles–Containing Orthodontic Primer. An In Vitro Study. Int J Dent. 2023 9246297. [PubMed.]
- S A Abdul–Razzaq., M S Khalaf. Effect of Dentin Surface Pretreatment With Chitosan Nanoparticles on Immediate and Prolonged Shear Bond Strength of Resin Composite. An in Vitro Study, Dental Hypotheses. 2023;14(3):84–86. [Ref.]
- V T Perchyonok., S Grobler., S Zhang., A Olivier., T Oberholzer. Insights into chitosan hydrogels on dentine bond strength and cytotoxicity. Open Journal of Stomatology. 2013;03(01):75–82. [Ref.]
- F Alkhudhairy. Experimental and Chitosan–Infused Adhesive with Dentin Pretreated with Femtosecond Laser, Methylene Blue–Activated Low–Level Laser, and Phosphoric Acid, Photobiomodulation. Photomedicine, and Laser Surgery. 2024;42(10):634–642. [PubMed.]
- I D Silva., L C C Boaro., B V Muniz., K Cogo–Muller., F Gonçalves., W C Brandt. The impact of chitosan in experimental resin with different photoinitiator systems. Journal of the Mechanical Behavior of Biomedical Materials. 2024;150:106323. [PubMed.]
- I R Ziotti., V L Paschoini., S A M Corona., A E Souza–Gabriel. Chitosan–induced biomodification on demineralized dentin to improve the adhesive interface. Restor Dent Endod. 2022;47(3):e28. [PubMed.]
- 113. H E Bettiol., W F Vieira–Junior., F M França., F L Amaral., R T Basting. Bonding strategy of a universal adhesive system containing chitosan: influence on dentin permeability, and effect on adhesive layer micromorphology. Acta Odontol Latinoam. 2022;35(3):206–213. [PubMed] [PubMed.]
- M Cicciù., L Fiorillo., G Cervino. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar Drugs. 2019;17(7). [PubMed.]
- M A Saghiri., R S Saini., M S Kuruniyan., S A Mosaddad., A Heboyan. Graphene and its modifications for enhanced adhesion in dental restoratives: a molecular docking and dynamics study, Scientific Reports. 2025;15(1):9455. [PubMed.]
- R Pereira., R B Lins., E F Lima., M D Mainardi., S Stamboroski., K Rischka., F H Aguiar. Properties of a Dental Adhesive Containing Graphene and DOPA–Modified Graphene, Polymers. 2024. [PubMed.]
- A G Williams., E Moore., A Thomas., J A Johnson. Graphene–Based Materials in Dental Applications: Antibacterial, Biocompatible, and Bone Regenerative Properties. Int J Biomater. 2023;8803283. [PubMed.]
- A Bregnocchi., E Zanni., D Uccelletti., et al. Graphene–based dental adhesive with anti–biofilm activity. Journal of Nanobiotechnology. 2017;15(1):89. [PubMed.]
- M Bin–Shuwaish., A Maawadh., R Al–Hamdan., et al. Influence of Graphene Oxide filler content on the Dentin bond integrity, Degree of conversion and bond strength of Experimental Adhesive, A SEM, Micro–Raman, FTIR and Microtensile study, Materials Research Express. 2020;7. [Ref.]
- Y F AlFawaz., B Almutairi., H F Kattan., M S Zafar., et al. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano–Particles–An SEM, EDX, Micro–Raman, and Microtensile Bond Strength Study, Polymers (Basel). 2020;12(12). [PubMed.]
- B–S M M., et al. Influence of graphene oxide filler content on the dentin bond integrit y, degree of conversion and bond strength of experimental adhesive. A SEM, micro–Raman, FTIR and microtensile study, Materials Research Express. 2020. [Ref.]
- A Y A Basil., F K Hiba., S Z Muhammad., F I N., M V Fahim., A T Dentin Bond. Integrity of Hydroxyapatite Containing Resin Adhesive Enha nced with Graphene Oxide Nano–Particles—An SEM, EDX, Micro–Raman, and Microtensile Bond Strength Study, Polymers. 2020;12(12):2978. [PubMed.]
- W Gamal., S Alrafee., A Sayed. The effect of incorporating graphene oxide nanoparticles within self–etch adhesive on the antibacterial properties and shear bond strength. 2022;68:3740. [Ref.]
- A Basil., F K Hiba., B Abdulelah., Q O I B Ghadeer., S A Aws., F I V Fahim. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion ., A scanning electron microscopy, energy dispersive X‐ray spectroscopy , Fourier transform infrared, micro‐Raman, and bond strength study, Microscopy research and technique. 2021. [Ref.]
- M L W Hou., W Cheng., X Huang. Graphene Oxide–Silica Composite Fillers into the Experimental. Dental A dhesives for Potential Therapy. 2017. [Ref.]
- S Kriplani., S Sedani. Comparative evaluation of the effect of 2% graphene oxide and 5% hydro xyapatite nanoparticles in isolation and in combination on micro tensi le bond strength of 5th generation adhesive, F1000Res. 2023;514. [Ref.]
- A Hanan., A Y A., E F., I V Fahim. Influence of carbon and graphene oxide nanoparticle on the adhesive pr operties of dentin bonding polymer: A SEM, EDX, FTIR study, Journal of Applied Biomaterials & Functional Materials. 2023. [PubMed.]
- B Almutairi., H F Kattan., A M BinMahfooz., O A Qutub., G Basunbul., et al. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion., A scanning electron microscopy, energy dispersive X–ray spectroscopy, Fourier transform infrared, micro–Raman, and bond strength study, Microsc Res Tech. 2021;84(9):2082–2094. [PubMed.]
- S Liu., A El–Angbawi., V Rosa., N Silikas. Physico–Mechanical Properties and Bonding Performance of Graphene–Added Orthodontic Adhesives. Journal of Functional Biomaterials. 2024. [PubMed.]
- AR Koltonow., C Luo., J Luo., J Huang. Graphene Oxide Sheets in Solvents: To Crumple or Not To Crumple?, ACS Omega. 2017;2(11):8005–8009. [PubMed.]
- A Tounici., J M Martín–Martínez. Addition of Graphene Oxide in Different Stages of the Synthesis of Waterborne Polyurethane–Urea Adhesives and Its Influence On Their Structure, Thermal. Viscoelastic and Adhesion Properties, Materials (Basel). 2020;13(13). [PubMed.]
- X Li X Liang., Y Wang., D Wang., M Teng., H Xu B Zhao., L Han. Graphene–Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook, Frontiers in Bioengineering and Biotechnology. 10 (2022) 804201. [Ref] [PubMed.]