>Corresponding Author : Han S Kim
>Article Type : Diagnostic challenge
>Volume : 5 | Issue : 1
>Received Date : 14 March, 2025
>Accepted Date : 24 March, 2025
>Published Date : 5 April, 2025
>DOI : https://doi.org/10.54289/JDOE2500103
>Citation : Kim HA, Hawie JB, Stancoven BW, Inouye KA, Lincicum AR, et al. (2025) Unilocular Radiolucency Between Mandibular Premolar Roots. J Dent Oral Epidemiol 5(1): doi https://doi.org/10.54289/JDOE2500103
>Copyright : © 2025 Kim HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Diagnostic challenge | Open Access | Full Text
1Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Eisenhower, Georgia, USA
2Department of Oral Pathology, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Eisenhower, Georgia, USA
*Corresponding author: Han S Kim, Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Eisenhower, Georgia, USA
Abbreviations: PDs: Probing Depths, CBCT: Cone-Beam Computed Tomography, LPC: Lateral Periodontal Cyst, BOC: Botryoid Odontogenic Cyst, OKC: Odontogenic Keratocyst, LRC: Lateral Radicular Cyst, COC: Calcifying Odontogenic Cyst.
The Challenge
A 63-year-old male presented to the Department of Periodontics, Army Postgraduate Dental School, Fort Eisenhower, Georgia, seeking sinus augmentation and implant therapy in the maxillary left posterior sextant. His medical history was significant for hypertension, high cholesterol, arthritis, and depression, along with a documented penicillin allergy. Extraoral and intraoral examinations showed no evidence of swelling, lymphadenopathy, or other abnormality. Probing depths (PDs) were three millimeters or less generally, with isolated four- to six-millimeter PDs limited to the molar areas. After nonsurgical periodontal therapy, the only residual five-millimeter PDs were found at the mesial line angles of the mandibular left first molar (Figure 1). A review of the patient’s radiographs revealed a wide, shallow infrabony defect at the mesial aspect of the mandibular first molar, incipient cupping of the interproximal alveolar bone in the remainder of the sextant, and an ovoid radiolucency between the mandibular left premolars midway between the interproximal alveolar crest and the apices of the teeth (Figure 2). Both mandibular left premolars were pristine teeth with no restorations or caries experience. Both teeth exhibited normal sensibility when challenged with a thermal stiumulus.
A cone-beam computed tomography (CBCT) scan revealed a corticated, ovoid, hypodense lesion measuring 3.0 × 2.4 mm between the roots of the mandibular left premolars (Figure 3). In cross section, the lesion measured 2.7 mm in the orofacial dimension and was positioned immediately deep to the buccal cortex. Both mandibular left premolars demonstrated normal sensibility.
The patient consented to biopsy of the intraosseous lesion in conjunction with open flap debridement at sites demonstrating residual five-millimeter PDs. Following administration of local anesthesia, full-thickness mucoperiosteal flaps were reflected in the mandibular left molar region. The buccal flap extended anteriorly to the canine to permit access to the intraosseous lesion. First molar root surfaces were thoroughly debrided with ultrasonic and hand instruments. The thin cortical bone overlying the lesion was carefully removed (Figure 4).
The lesion readily separated from the surrounding bone, and the specimen (Figure 5) was sent to the Department of Oral Pathology for histopathological analysis. Normal healing was noted at all follow-up appointments.
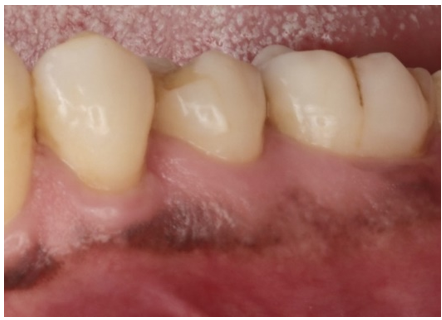
Figure 1: Clinical appearance of the mandibular left posterior sextant.
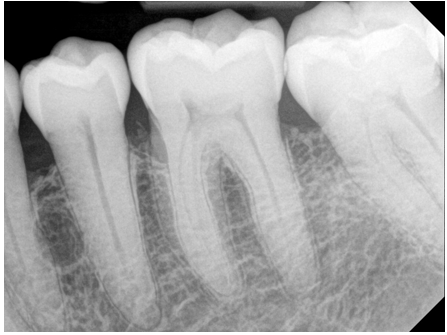
Figure 2: Periapical radiograph. An ovoid radiolucency was noted between the roots of the mandibular left premolars.
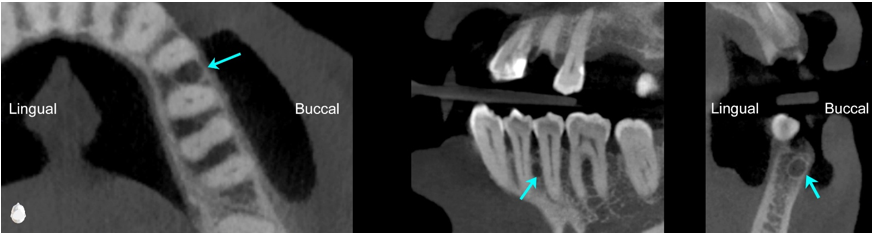
Figure 3: Axial, custom, and cross-sectional images from the cone beam computed tomography volume. An ovoid, corticated, hypodense lesion (3.0 x 2.4 mm) was identified between the roots of the mandibular left premolars (arrows).
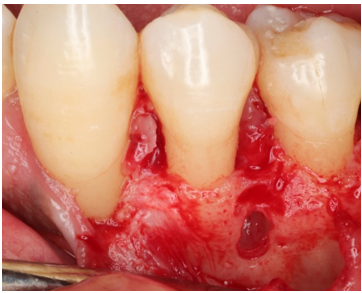
Figure 4: Surface of the lesion after removal of the overlying cortical bone.
Figure 5: Specimen sent for histopathological analysis.
Histopathological examination under low power magnification revealed a fragmented cyst wall composed of fibrous connective tissue surfaced by a detached, stratified squamous epithelial lining (Figure 6). Medium-power magnification revealed nodular epithelial plaques containing glycogen-rich clear cells within the cyst lining (Figure 7). High-power magnification of the remaining detached cyst lining demonstrated a uniform, non-keratinized, thin stratified squamous epithelium (Figure 8).
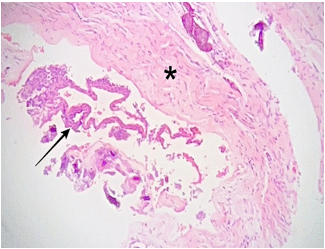
Figure 6: Photomicrograph, hematoxylin and eosin staining. Low power view (5x magnification) showing a fragmented, fibrous connective tissue cyst wall (*), and detached stratified squamous epithelium cyst lining (arrow)
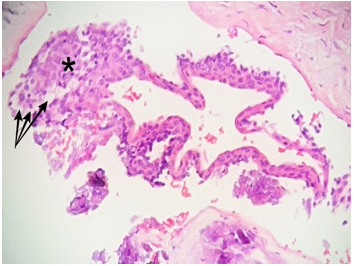
Figure 7: Photomicrograph, hematoxylin, and eosin staining. Medium power view (10x magnification) of the detached cyst lining shows a nodular epithelial plaque (*) with characteristic, glycogen containing, clear cells (arrows)
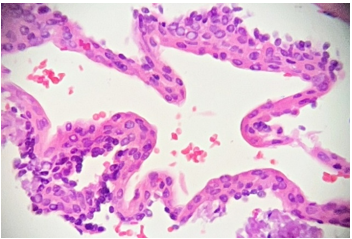
Figure 8: Photomicrograph, hematoxylin, and eosin staining. High power view (20x magnification) of the detached cyst lining highlights the uniform nature of the non-keratinized, thin stratified squamous epithelium.
The Diagnosis
C. Lateral Periodontal Cyst
The lateral periodontal cyst (LPC) is a rare developmental odontogenic cyst thought to arise from remnants of the dental lamina [1-4]. The majority of LPCs occur in the mandibular premolar-canine-lateral incisor area, and the lesions are usually asymptomatic [1]. When a LPC is found in the maxilla, the lateral incisor area is the most common location [2]. Historically, the term LPC was applied to any cyst occurring along the lateral root surface of a tooth; however, LPC is a distinct pathological entity with distinguishing microscopic features [1].
The lesion usually appears in patients aged 40 to 80 years as a well-demarcated, unilocular round or pear-shaped radiolucency between the roots of vital teeth, which may exhibit splaying [1-5]. Notably, it is possible for teeth adjacent to LPCs to become nonvital due to a process unrelated to cyst development, such as trauma or caries [2]. Rarely, the lesion may appear as a polycystic variant, called botryoid odontogenic cyst (BOC), which may have a multilocular or unilocular radiographic appearance [1-5]. LPC can be radiographically indistinguishable from other odontogenic lesions such as the odontogenic keratocyst (OKC), lateral radicular cyst (LRC), and calcifying odontogenic cyst (COC). Thus, histopathologic evaluation is necessary to confirm the diagnosis. LPCs are characteristically lined by a nonkeratinized simple or stratified squamous/cuboidal epithelium with a thickness of only a few cell layers—a feature necessary for LPC diagnosis [1-4]. Nodular epithelial thickenings or plaques are sometimes found within the otherwise thin cyst lining, and
when present, are helpful in distinguishing the lesion [2]. Epithelial rests within the connective tissue lining may be present but are not essential for LPC diagnosis [2]. LPCs—particularly BOC variants—are thought to retain limited neoplastic growth potential [2]. Treatment for LPC is usually conservative enucleation, and recurrence is unusual [1]. One case of squamous cell carcinoma apparently occurring in a LPC has been reported [1].
In this case, the well-circumscribed radiolucency occurred in the most common location for LPC. The patient was asymptomatic, and the lesion produced slight displacement of the mandibular left premolar roots. Nevertheless, diagnosis relied upon the histopathologic assessment.
Differential Diagnoses
Odontogenic Keratocyst
OKCs are more common than LPCs, the lesions accounting for 3% to 11% and less than 2% of odontogenic cysts, respectively [1]. Like LPCs, OKCs derive from rests of the dental lamina [1]. However, these two entities exhibit very different clinical behavior. Indeed, the World Health Organization currently classifies the OKC as an odontogenic cyst, although some experts favor grouping the lesion with odontogenic tumors under the term keratocystic odontogenic tumor based predominantly on genetic analyses [1,6,7]. OKCs exhibit aggressive growth potential, a high recurrence rate, and possible association with nevoid basal cell carcinoma syndrome, which should be suspected when multiple OKCs are present [6,8]. These lesions tend to grow in an anteroposterior direction within the jaw, without clinically obvious bony expansion [1]. Like LPCs, OKCs are more common in the mandible than the maxilla. However, OKCs exhibit strong predilection for the posterior mandibular body and ramus rather than the premolar-canine-lateral incisor area [1]. Although an OKC may be identified at any age, they are typically diagnosed earlier than LPCs, 60% of lesions occurring in the second and third decades of life [1,2]. In contrast, LPCs are rarely diagnosed before age 30 [1]. Both OKCs and LPCs are more common in males [1,2]. For LPCs, the male to female ratio is 2:1, whereas OKCs exhibit only a slight male predilection [1,2]. A small OKC is often asymptomatic and discovered as an incidental radiographic finding. Larger OKCs may be asymptomatic or present with pain, swelling, and discharge [1].
OKC could not be ruled out in this case based on clinical and radiographic findings. However, the histopathological assessment did not support OKC diagnosis. The diagnostic feature of OKC is a corrugated parakeratinized epithelial lining [9]. which was absent from this specimen. In OKCs, the epithelium is usually [6-8] cell layers thick, and low columnar or elongated basal cells with hyperchromatic nuclei are palisaded at the basement membrane [9,10]. The lesion may demonstrate a unicystic or more complex architecture [10]. The lumen typically contains thick keratin debris, although solid OKC variants have been reported [10,11]. Satellite cysts and/or epithelial rests are present in the fibrous OKC wall in about 20% of cases [10]. These are more common in syndrome-associated rather than solitary OKCs [10]. Rushton bodies (linear or arch-shaped calcifications), cholesterol clefts, and mucous cells are found less frequently [10]. OKC treatment is enucleation and curettage. A reasonable estimate for the recurrence rate is 21% to 30%, and some experts recommend measures such as peripheral ostectomy and use of Carnoy’s solution to reduce recurrence [1].
Lateral Radicular Cyst
In contrast to LPCs and OKCs, which are developmental cysts, LRCs are inflammatory cysts of odontogenic origin—variants of periapical cysts that appear along the lateral surface of a root rather than the apex [1,3,10]. In perspective, inflammatory lesions associated with nonvital teeth represent the most frequently encountered radiolucent pathology affecting the tooth-bearing regions of the jaws [10,12,13]. Epithelial rests of Malassez represent the usual source of epithelium stimulated by the inflammation, although other epithelial sources, such as the lining of the Schneiderian membrane, are possible [1]. LRCs may produce pain and swelling or remain asymptomatic [3,10]. In the presented case, LRC could be ruled out clinically. There was no history of trauma. Both adjacent teeth were unrestored, with normal responses to thermal challenge and no evidence of caries. Thus, the lesion lacked association with a nonvital tooth—a defining LRC characteristic
Histologically, LRC is characterized by a stratified squamous epithelium [2-3] cell layers thick [1,10]. However, the epithelium may demonstrate hyperplasia, spongiosis, and exocytosis in the presence of a robust inflammatory response [1,10]. The lumen is typically filled with cellular debris and fluid, and an acute or chronic inflammatory infiltrate is typically present in the fibrous wall of the cyst [1,10]. As described for OKCs, Rushton bodies—a nonspecific finding in inflamed odontogenic cysts—may be present [10]. Treatment of a LRC usually involves nonsurgical root canal therapy or tooth extraction [1]. A residual cyst may persist after treatment, and in such cases, biopsy is recommended to rule out pathological processes that mimic inflammatory lesions [1].
Calcifying Odontogenic Cyst
The COC exists within a spectrum of intraosseous ghost cell odontogenic lesions for which the classification remains unclear [1,14,15]. The lesions are cystic (COC), solid (dentinogenic ghost cell tumor), or malignant (ghost cell odontogenic carcinoma) [1]. Whether these are distinct entities or variable manifestations of the same pathologic process is uncertain, although separation of cystic from solid lesions appears appropriate [1,2,14,15]. Like LPC, OKC, and LRC, the COC epithelium derives from the dental lamina, which is odontogenic [1,2]. Peak incidence of COC falls within the second and third decades of life [2]. The lesion can occur in any region of the jaws, 65% of lesions occurring in the incisor-canine areas [1]. A slight predominance within the maxillary arch has been noted, with no significant sex predilection [2,14].
Because of its tendency to involve products of odontogenesis, the COC is considered a mixed radiolucent-radiopaque lesion, although most examples are radiolucent unless associated with an odontoma [2]. COCs may appear as well-circumscribed unilocular or multilocular lesions that may resorb or displace roots of adjacent teeth [1,2]. Ghost cells can occur in multiple types of odontogenic cysts and are not pathognomonic for COC [2]. However, the epithelial lining of the cyst has been described as ameloblastoma-like with focal accumulations of ghost cells, which are capable of calcification [9,15]. The epithelial lining varies in thickness, with a palisading columnar basal cell layer [15]. Recurrence of COC is low after simple enucleation, and with treatment the patient has a good prognosis [1]. In contrast, solid forms of odontogenic ghost cell tumors are potentially aggressive and show substantial recurrence following aggressive treatment [1].
In this case, the lesion demonstrated a purely radiolucent radiographic appearance and produced slight displacement of adjacent roots. Neither of these observations are inconsistent with COC. However, the posterior location of the lesion rendered COC less likely. The histologic analysis did not show diagnostic features of COC such as ameloblastoma-like epithelium, ghost cells, and products of odontogenesis.
Conclusion
Although inflammatory lesions are by far the most common radiolucent pathologic entities affecting the tooth-bearing regions of the jaws, clinical and radiographic features of the lesion in the presented case made LRC highly unlikely. Multiple developmental odontogenic cysts and tumors can appear radiographically identical to the encountered lesion. Based on the location of occurrence and slight splaying of adjacent tooth roots, it was reasonable to suspect LPC. However, definitive diagnosis relied upon histopathologic evaluation. Among the most likely pathologic processes, prognosis and likelihood of recurrence varied considerably. Obtaining and preserving a specimen that permits a proper histopathologic assessment is critical for optimizing clinical and patient-oriented outcomes.
Funding: The Defense Health Agency funded the treatment documented in the presented case. The authors received no extramural funding.
Disclaimer: The views and opinions expressed in this manuscript are solely those of the authors and do not reflect the official policy or position of the United States Government, the Department of Defense, the Defense Health Agency, or Uniformed Services University.
Conflict of interests: The authors report no financial, economic, or professional interests that may have influenced the design, execution, or presentation of this work.
Author contribution statement
Conceptualization: Han Kim, Brian Stancoven, Kimberly Ann Inouye, Adam Lincicum
Investigation/treatment: Han Kim
Methodology: Thomas Johnson
Formal analysis: Jennifer Hawie
Writing–original draft: Han Kim, Thomas Johnson, Jennifer Hawie
Writing–review & editing: Brian Stancoven, Kimberly Ann Inouye, Adam Lincicum
References
- Neville BW., Damm DD., Allen CM., Chi AC. Odontogenic cysts and tumors. In Oral and Maxillofacial Pathology. 5th ed, Elsevier. 2024:685-746. [Ref.]
- Hellstein JW., Timmons SR. Odontogenesis Odontogenic Cysts and Odontogenic Tumors. In Cummings Otolaryngology Head and Neck Surgery. Elsevier. 2021:1254-1275. [Ref.]
- Eliasson S., Isacsson G., Köndell PA. Lateral periodontal cysts. Clinical, radiographical and histopathological findings. Int J Oral Maxillofac Surg. 1989;18(4):191-193. [PubMed.]
- Woo SB., Odontogenic cysts. In Oral Pathology. 3rd ed. Elsevier. 2024:414-448. [Ref.]
- Chrcanovic BR., Gomez RS. Gingival cyst of the adult, lateral periodontal cyst, and botryoid odontogenic cyst: An updated systematic review. Oral Dis. 2019;25(1):26-33. [PubMed.]
- Soluk-Tekkesin M., Wright JM. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2022 (5th) Edition. Turk Patoloji Derg. 2022;38(2):168-184. [PubMed.]
- Vered M., Wright JM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors. Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022;16(1):63-75. [PubMed.]
- Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002;38(4):323-331. [PubMed.]
- Speight PM., Hunter KD. Tumors of the oral cavity. In Diagnostic Histopathology of Tumors. Elsevier. 2021:273-300. [Ref.]
- McLean AC., Vargas PA. Cystic Lesions of the Jaws: The Top 10 Differential Diagnoses to Ponder. Head Neck Pathol. 2023;17(1):85-98. [PubMed.]
- Vered M., Buchner A., Dayan D., Shteif M., Laurian A. Solid variant of odontogenic keratocyst. J Oral Pathol Med. 2004;33(2):125-128. [PubMed.]
- Becconsall-Ryan K., Tong D., Love RM. Radiolucent inflammatory jaw lesions: a twenty-year analysis. Int Endod J. 2010;43(10):859-865. [PubMed.]
- Koivisto T., Bowles WR., Rohrer M. Frequency and distribution of radiolucent jaw lesions: a retrospective analysis of 9,723 cases. J Endod. 2012 Jun;38(6):729-732. [PubMed.]
- Ledesma-Montes C., Gorlin RJ., Shear M., et al. International collaborative study on ghost cell odontogenic tumours: calcifying cystic odontogenic tumour, dentinogenic ghost cell tumour and ghost cell odontogenic carcinoma. J Oral Pathol Med. 2008;37(5):302-308. [PubMed.]
- John S., Devi P., Sharma K., Sankar R., Gupta S. Calcifying odontogenic cysts: A novel outlook on classification, diagnosis and management. Semin Diagn Pathol. 2024:S0740-2570(24)00072-8. [PubMed.]