>Corresponding Author : Daniel A DiPirro
>Article Type : Original Research Article
>Volume : 5 | Issue : 2
>Received Date : 14 March, 2025
>Accepted Date : 31 March, 2025
>Published Date : 03 September, 2025
>DOI : https://doi.org/10.54289/JDOE2500102
>Citation : DiPirro DA, Boudreaux DM, Ange BL and Johnson TM. (2025) Influence of Neodymium-Doped Yttrium Aluminum Garnet Laser Irradiation on Cell Proliferation and Bone Morphogenetic Protein-2 Secretion in MG-63 Osteoblast-like Cells. J Dent Oral Epidemiol 5(2): doi https://doi.org/10.54289/JDOE2500102
>Copyright : © 2025 DiPirro DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Original Research Article | Open Access | Full Text
1Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Cavazos, Texas, USA
2Department of Clinical Investigation, Dwight David Eisenhower Army Medical Center, Fort Eisenhower, Georgia, USA
3Department of Surgery, Medical College of Georgia, Augusta University, Augusta, Georgia, USA
4Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Eisenhower, Georgia, USA
*Corresponding author: Daniel A DiPirro, Department of Periodontics, Army Postgraduate Dental School, Postgraduate Dental College, Uniformed Services University, Fort Cavazos, Texas, USA
Abstract
The aim of this in vitro study was to assess the influence of neodymium-doped yttrium aluminum garnet (Nd:YAG) laser irradiation on proliferation and bone morphogenetic protein-2 (BMP-2) secretion in MG-63 osteoblast-like cells. MG-63 cells were cultured in plastic 96-well plates and exposed to Nd:YAG laser irradiation (1064 nm). Independent variables included power (0.25 to 5.0 W) and irradiation time (10 to 60 s). In test and control groups, BMP-2 secretion was assessed using two methods: enzyme-linked immunosorbent assay (ELISA) and magnetic microsphere BMP-2 immunoassay. In addition, cell viability and proliferation were evaluated using water-soluble tetrazolium (WST-1) and cell counting kit-8 (CCK-8) assays. Outcome variables were assessed 24 and 72 h following laser exposure. Mann-Whitney U tests and Kruskal-Wallis tests were applied to assess differences in cell cultures across the independent variables of interest. Magnetic microsphere immunoassay analysis failed to identify any statistically significant influence of laser energy on BMP-2 secretion irrespective of power or irradiation time. In the ELISA analysis, BMP-2 concentration was significantly higher for irradiation time of 10 versus 60 seconds (p=0.009) at the 24-hour assessment only. CCK-8 analysis showed that, compared with control cultures, cell proliferation was significantly higher at the 24-hour recording for cells receiving power of 0.25 W (p=0.0048), 1 W (p=0.0048), 3 W (p=0.0048), or 5 W (p=0.0048). Similar effects on cell proliferation were noted at the 24- and 72-hour assessments. Under the conditions described, Nd:YAG laser irradiation consistently increased proliferation of MG-63 cells. However, in almost all experiments, BMP-2 secretion in control and laser-treated cell cultures were not statistically different. Differences in target cell types and laser irradiation parameters likely account for discordance between findings of the present investigation and those reported in prior studies.
Keywords: Lasers, Bone Morphogenetic Proteins, Bone Regeneration, Osteoblasts, Cell Proliferation, Cell Culture Techniques
Abbreviations: ND YAG: Neodymium-Doped Yttrium Aluminum Garnet, BMP: Bone Morphogenetic Protein, ELISA: Enzyme-Linked Immunosorbent Assay, WST: Water-Soluble Tetrazolium, CCK: Cell Counting Kit, TGFB: Transforming Growth Factor-Β, ALP: Alkaline Phosphatase, DMEM: Dulbecco Modified Eagle Medium, MFI: Median Fluorescence Intensity, ELISAs: Enzyme-Linked Immunosorbent Assays, DSCF: Dwass, Steel, Critchlow-Fligner
Introduction
In 1965, Dr. Marshall Urist, an orthopedic surgeon, made the landmark discovery that devitalized bone implanted into animal soft tissue induces cellular responses culminating in ectopic bone formation [1]. Subsequently, the proteins predominantly responsible for this effect—termed “bone morphogenetic proteins” (BMPs)—were identified, purified, and amplified through molecular cloning techniques [2,3]. BMPs comprise a group of related dimeric proteins belonging to the transforming growth factor-β (TGFβ) superfamily [3]. When implanted in vivo, BMPs induce mesenchymal cell infiltration and differentiation, a transient cartilage phase, and endochondral ossification followed by normal bone remodeling [3]. In animal models, multiple assessments of bone formation using BMP-2 and space provision have demonstrated regenerates histologically similar to immediately adjacent native bone [4,5].
In clinical case reports, neodymium-doped yttrium aluminum garnet (Nd:YAG, 1064 nm) lasers have been used to establish stable blood clots at alveolar ridge preservation and immediate implant sites [6-8]. However, use of Nd:YAG lasers for regenerative periodontal therapy remains controversial, and to date, studies elucidating the biologic basis and clinical predictability of such treatments are lacking [9,10]. Nevertheless, investigators have suggested that Nd:YAG laser energy may induce favorable cellular effects supportive of bone and periodontal regeneration. In particular, some evidence suggests that near infrared laser energy may upregulate BMP-2 gene expression and protein secretion in cells of the osteoblast lineage. Kim and colleagues exposed mouse osteoblast precursor cell (MC3T3-E1) cultures to Nd:YAG laser irradiation at various energy densities [11]. Laser stimulation significantly increased BMP-2 gene expression and protein levels [11]. Furthermore, both Nd:YAG laser exposure and exogenous BMP-2 application increased expression of BMP-2 responsive transcription factors Cbfa1 and Osterix [11]. Another group of investigators irradiated cultures of a human fetal osteoblast cell line using a gallium aluminum arsenide (Ga-Al-As) diode laser (808 nm) under hypoxic conditions [12]. Utilizing quantitative real-time polymerase chain reaction, the authors concluded that laser-treated cells exhibited significantly increased BMP-2 expression compared with controls [12]. Fujimoto and coworkers investigated the effect of Ga-Al-As diode laser energy on mouse osteoblast precursor cell (MC3T3-E1) cultures [13]. Laser exposure significantly increased BMP expression and transcription factors associated with osteoblast differentiation [13]. Use of the BMP receptor blocker Noggin inhibited the laser-induced transcription factor expression [13]. The same laboratory found, in another in vitro study, that rat calvarial osteoblast-like cells stimulated by Ga-Al-As laser irradiation exhibited increased cellular proliferation, bone nodule formation, and alkaline phosphatase (ALP) expression/activity [14]. The purpose of the present investigation was to assess proliferation and BMP-2 protein concentrations in human MG-63 osteoblast-like cell cultures after exposure to Nd:YAG laser irradiation.
Materials and Methods
A schematic of the experimental design is shown in (Figure 1). All experiments were conducted in a sterile laminar flow hood on a non-reflective surface.
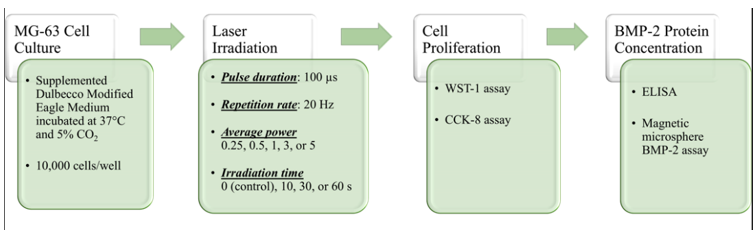
Figure 1: Experimental design. Cell proliferation and BMP-2 concentration were evaluated at two time points: 24 and 72 h following laser exposure.
Cell Culture
MG-63 osteoblast-like cells were cultured in supplemented Dulbecco Modified Eagle Medium (DMEM, Thermo Fisher Scientific, Waltham, Massachusetts, USA) incubated at 37°C and 5% CO2 in a humidified atmosphere. At confluence, cells were harvested and plated in 96-well plates at a density of 10,000 cells per well. A checkerboard pattern was utilized on the 96-well plate to protect adjacent samples from inadvertent laser exposure. The cultures then incubated for 24 hours under the described conditions.
Laser Irradiation
Nd:YAG laser energy (1064 nm, Lightwalker AT, Fotona, Dallas, Texas, USA) was delivered to the cell cultures in 96-well plates using a 300-µm diameter optical fiber stabilized 3 cm from the plate, such that the aiming beam spot size matched the area of a single well (0.32 cm2). Average power was set at 0 (control), 0.25, 0.5, 1, 3, or 5 W, with an exposure time of 10, 30, or 60s. Repetition rate and pulse duration were constant at 20 Hz and 100 µs, respectively (Table 1). Control cultures were removed from the incubator for the duration of laser irradiation. Laser exposure groups received Nd:YAG laser irradiation 24 h after plating cells.
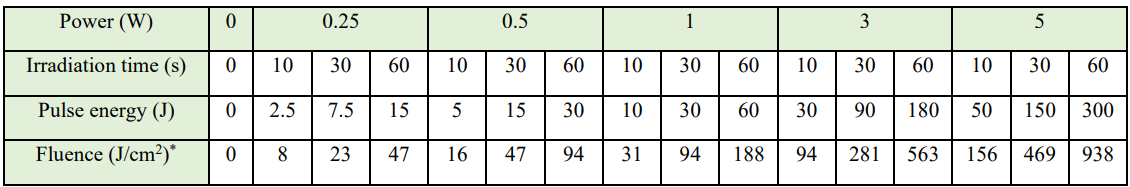
Table 1: Nd:YAG laser irradiation parameters
*The irradiated surface area consisted of a single well in a 96-well plate, measuring 0.32 cm2. We achieved this condition by stabilizing a 300-µm fiber 3 cm from the cell culture.
Bone Morphogenetic Protein-2 Protein Levels
Magnetic Microsphere BMP-2 Immunoassay: A magnetic microsphere immunoassay (MAGPIX System, Luminex, Austin, Texas, USA) was used to detect BMP-2 protein concentrations in the cell culture supernatant. BMP-2-specific antibodies were pre-coated onto magnetic microspheres embedded with fluorophores. Microspheres and samples from the cell supernatant were applied to 96-well microplates using a pipette. The samples were read using a magnetic microsphere analyzer (MAGPIX System, Luminex). The microspheres were magnetically positioned in a monolayer and identified based on fluorescent patterns. A green LED then illuminated the microspheres, and a CCD camera detected the resulting fluorescence. The median fluorescence intensity (MFI) provided the basis for the sample analysis. The analysis software (MAGPIX System, Luminex) processed the images and determined BMP-2 concentrations in picograms per milliliter (pg/ml) using a standard curve.
Enzyme-Linked Immunosorbent Assay: Enzyme-linked immunosorbent assays (ELISAs) were used to determine BMP-2 concentrations occurring in cell supernatant. Using a pipette, cell samples were applied to 96-well microplates pre-coated with a BMP-2-specific monoclonal antibody. An enzyme-linked, BMP-2-specific monoclonal antibody was added to the wells. Absorbance at 450 nm (A450) was recorded using a microplate reader (Varioskan LUX multimode microplate reader, Thermo Fisher Scientific) within 30 minutes and converted to BMP-2 concentration using a standard curve.
Cell Proliferation Assays: Both water-soluble tetrazolium (WST-1) and cell counting kit-8 (CCK-8) cell proliferation assays relied upon cleavage of a tetrazolium salt by mitochondrial dehydrogenases to form formazan in viable, metabolically active cells. Formation of the formazan product increased the A450, as recorded using a microplate reader (Varioskan LUX multimode microplate reader, Thermo Fisher Scientific). The A450 was then converted to viable cell count using a standard curve.
Statistical Analysis
Statistical software (SAS 9.4, SAS Institute, Cary, North Carolina, USA) was used for all analyses, with significance level set at 0.05. Descriptive statistics were calculated for all qualitative and quantitative variables. The primary outcome variable was BMP-2 concentration, assessed via two methods—magnetic microsphere immunoassay and ELISA. Explanatory variables included assessment time (24 or 72 h), irradiation time (10 or 60 s), and power (0, 0.25, 1, 3, or 5 W). Individual analyses were conducted for each assessment time using non-parametric tests. Wilcoxon-Mann-Whitney U tests were calculated separately at each assessment (24 and 72 h following laser exposure) to determine if irradiation time (10 versus 60 s) influenced BMP-2 protein level. Next, Kruskal-Wallis tests were calculated at each assessment time to determine if BMP-2 concentration differed by laser power (0, 0.25, 1, 3, or 5 W). If the Kruskal-Wallis test was statistically significant, the Bonferroni adjustment was used to adjust the alpha level and individual Mann-Whitney U tests were calculated to compare BMP-2 concentrations in control versus laser-stimulated cultures.
In addition to BMP-2 secretion, cell proliferation was assessed using CCK-8 and WST-1 kits. Explanatory variables included assessment time (24 or 72 h), irradiation time (10, 30, or 60 s), and laser power (0, 0.25, 1, 3, or 5 W). Individual analyses were conducted for each assessment time. Due to the non-normal nature of the data, non-parametric tests were used. Kruskal-Wallis tests were used to determine if cell proliferation differed by irradiation time (10, 30, or 60 seconds) at the 24- and 72-h assessments. A Dwass, Steel, Critchlow-Fligner (DSCF) multiple comparison procedure was used to assess for multiple comparisons for irradiation time. If the Kruskal-Wallis test for laser power was statistically significant, the Bonferroni adjustment was used to adjust the alpha level and individual Mann-Whitney U tests were calculated to compare cell proliferation in control versus laser-stimulated cultures.
Results
BMP-2 Secretion
Magnetic microsphere immunoassay results. Table 2 presents the results of the Kruskal-Wallis tests calculated to determine if BMP-2 concentration differed by power and the results of Wilcoxon-Mann-Whitney U tests to determine if BMP-2 concentration differed by irradiation time. Neither power nor irradiation time produced a statistically significant difference in BMP-2 concentration under the described conditions.
ELISA results. Table 3 reports the results of the Kruskal-Wallis tests calculated to determine if BMP-2 concentration differed by power and the results of Wilcoxon-Mann-Whitney U tests to determine if BMP-2 concentration differed by irradiation time. No statistically significant difference in BMP-2 concentration by power at either assessment time or by irradiation time at the 72-hour assessment was observed (Figure 2). At the 24-hour assessment point, BMP-2 concentration exhibited a statistically significant elevation at the shorter (10 s) versus longer (60 s) irradiation time (p=0.009).
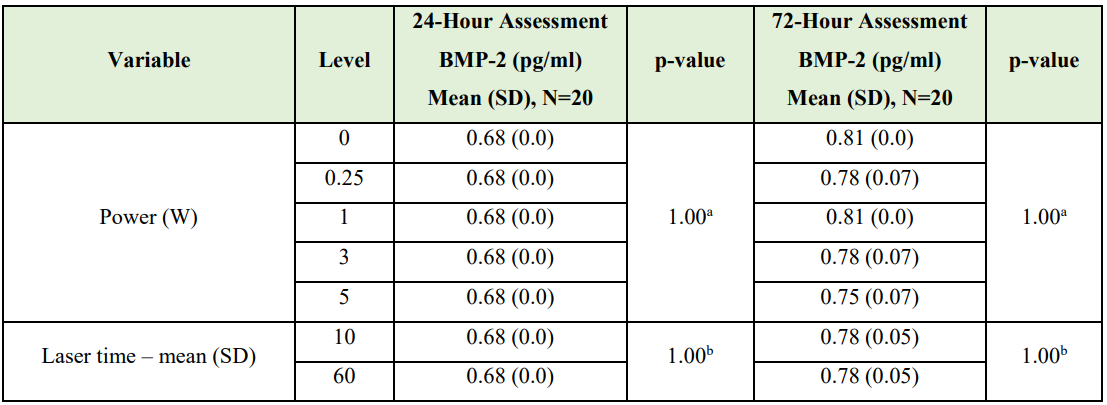
Table 2: Kruskal-Wallis / Wilcoxon-Mann-Whitney U Test Results
(BMP-2 Protein Concentration - Magnetic Microsphere Immunoassay)
aKruskal-Wallis test
bWilcoxon-Mann-Whitney U test
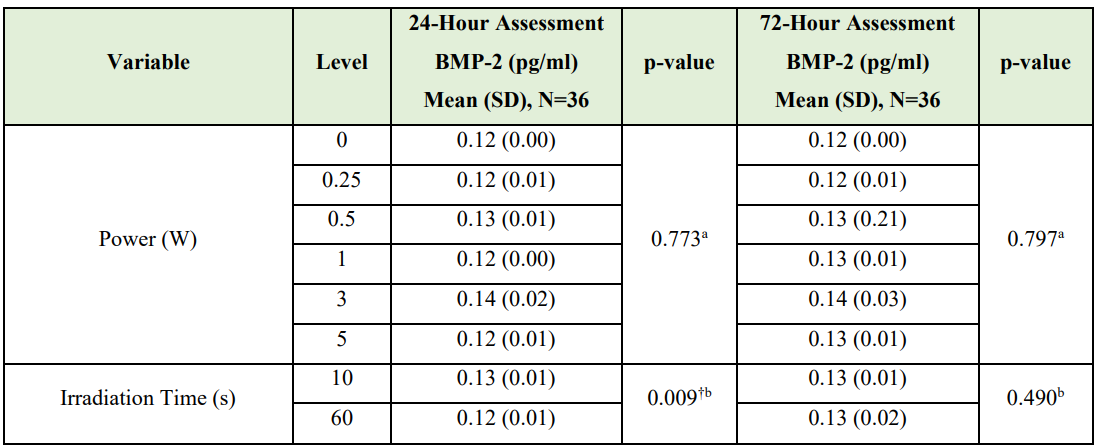
Table 3: Kruskal-Wallis / Wilcoxon-Mann-Whitney U Test Results
(BMP-2 Protein Concentrations - ELISA Dataset)
†Statistically significant
aKruskal-Wallis test
bWilcoxon-Mann-Whitney U test
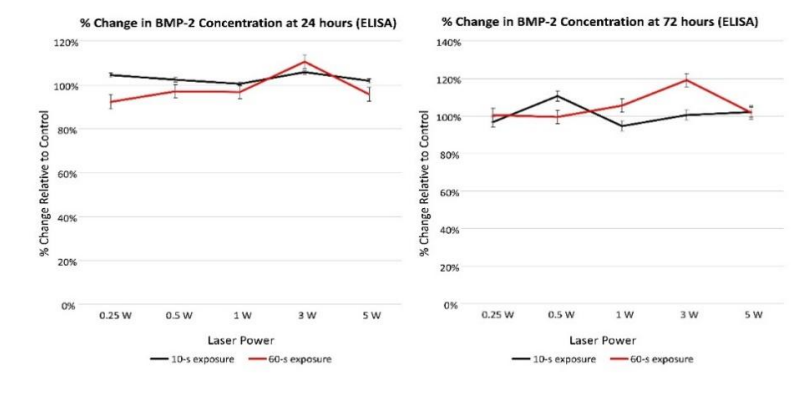
Figure 2: BMP-2 concentration (percent change relative to control) assessed using ELISA at 24 and 72 h following laser exposure.
Cellula Proliferation
CCK-8 data. The results of the Kruskal-Wallis test showed a statistically significant difference in cell proliferation by laser power at the 24- and 72-h assessment times (<0.0001 and 0.0014, respectively). Table 4 reports results of the Wilcoxon-Mann-Whitney U tests to determine if cell proliferation differed by irradiation time and power. Compared with control cultures, cell proliferation was significantly higher at the 24-h recording for cultures receiving 0.25 W (p=0.0048), 1 W (p=0.0048), 3 W (p=0.0048), 5 W (p=0.0048), or positive laser power (p=0.0046) after adjusting for multiple comparisons (alpha=0.01). At the 72-hour recording, cell proliferation was significantly higher than controls for cultures receiving 0.25 W (p=0.0048), 1 W (p=0.0048), 3 W (p=0.0048), 5 W (p=0.0046), or positive laser power (p=0.0046) after adjusting for multiple comparisons (alpha=0.01). CCK-8 proliferation data demonstrated up to 46% increase in cell proliferation 72 hours after Nd:YAG laser irradiation (Figure 3).
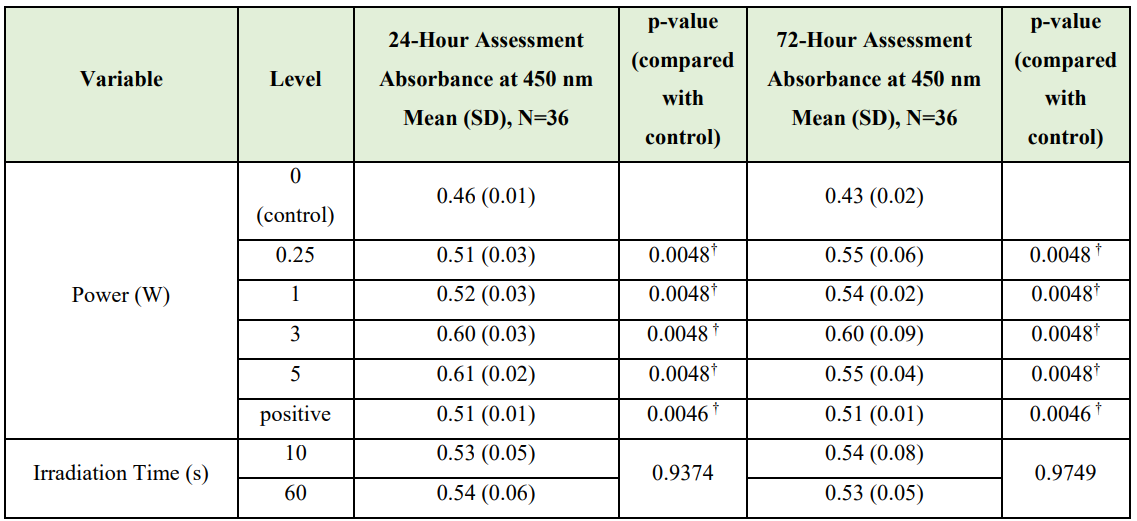
Table 4: Wilcoxon-Mann-Whitney U Test Results
(Cell Proliferation - CCK-8 Dataset)
OD450 = Optical density at 450 nm
†Statistically significant
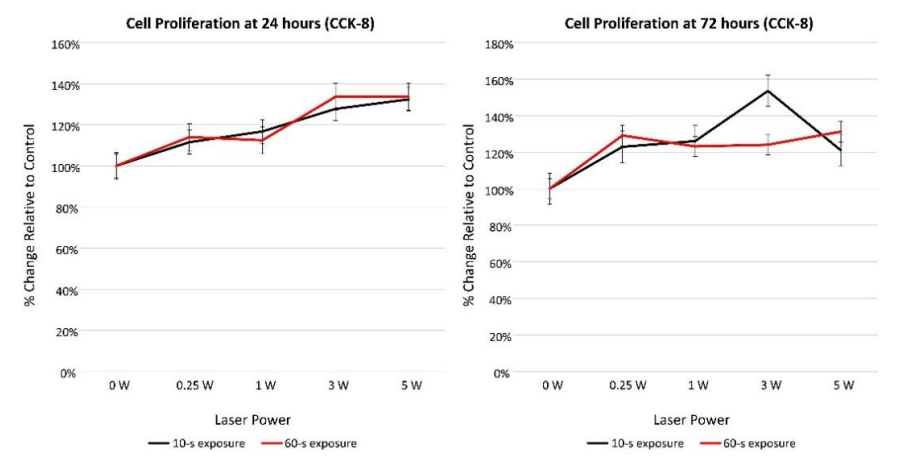
Figure 3: CCK-8 cell proliferation data (percent change relative to control) at 24- and 72-h assessments.
WST-1 data. The results of the Kruskal-Wallis test showed a statistically significant difference in cell proliferation by laser power at the 24- and 72-h assessment times (0.0016 and <.0001, respectively). Table 5 reports results of the Wilcoxon-Mann-Whitney U tests or Kruskal-Wallis test to determine if cell proliferation differed by irradiation time and power. Statistically significant differences in cell proliferation were identified, after adjusting for multiple comparisons (alpha=0.0017), when comparing control cultures with cells receiving power of 0.5 W (p=0.0004), 1 W (p=0.0005), and 5 W (p=0.0011). WST-1 proliferation data demonstrated up to 46% increase in cell proliferation 72 hours after Nd:YAG laser irradiation (Figure 4).
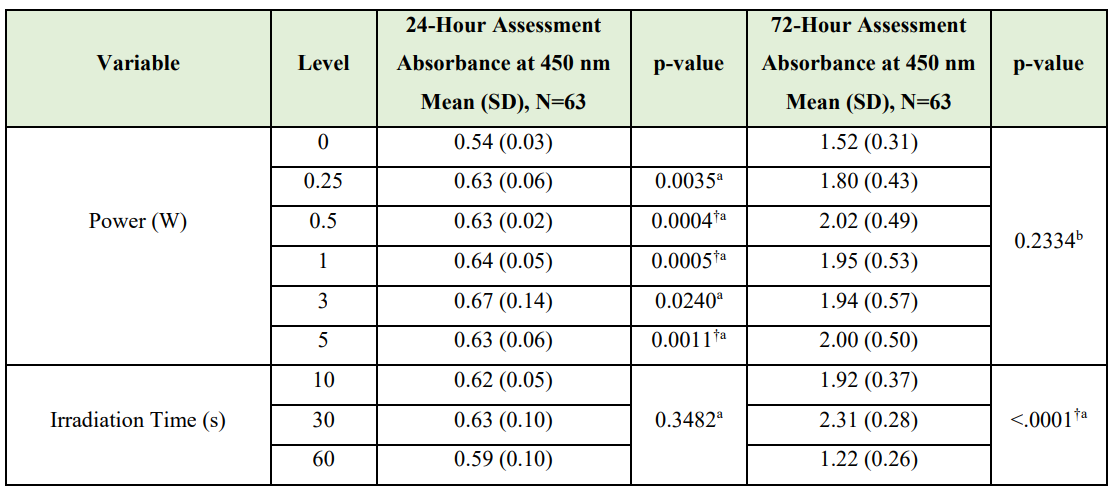
Table 5: Kruskal-Wallis / Wilcoxon-Mann-Whitney U Test Results
(Cell Proliferation – WST-1 Dataset).
aWilcoxon-Mann-Whitney U test
bKruskal-Wallis test
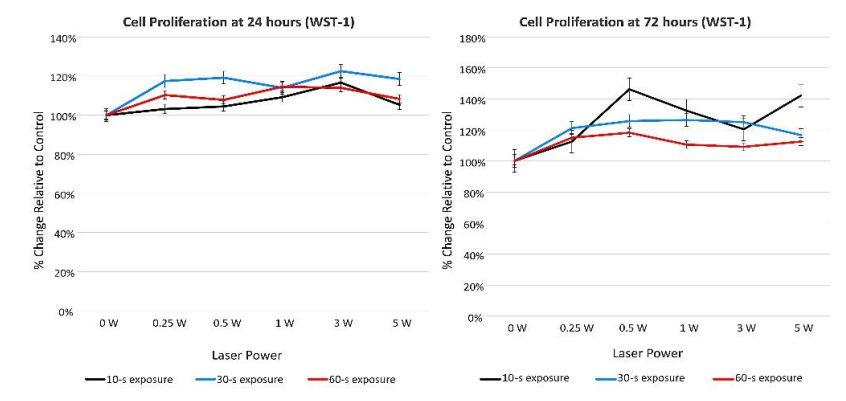
Figure 4: WST-1 cell proliferation data (percent change relative to control) at 24- and 72-h assessments.
Discussion
The purpose of this investigation was to assess the influence of Nd:YAG laser irradiation on cell proliferation and BMP-2 secretion in MG-63 osteoblast-like cells. Nd:YAG laser stimulation was found to consistently stimulate MG-63 cell proliferation over the range of energy densities applied. In some experiments, cell proliferation increased by 46% in laser-exposed versus control cultures (Figures 3 and 4). This finding is consistent with results of Karoussis and colleagues, who observed increased MG-63 cell proliferation on plastic and titanium surfaces following Nd:YAG laser irradiation (4 J/cm2), an effect enhanced by a roughened titanium substrate [15]. Increased cell proliferation has been a common finding in studies investigating various lasers, irradiation parameters, and target cell types [15-18]. Arisu and colleagues reported increased proliferation of Saos-2 cells in response to Nd:YAG laser exposure [16]. However, pulse energy, repetition rate, and power correlated negatively with cell proliferation [16]. This observation is an example of “biphasic dose response.” For many outcomes of interest—such as cell proliferation—an optimal laser exposure is definable. Maximum benefit may occur at the optimal dose, with inhibitory or even detrimental effects at higher exposure levels [17]. Producing the maximal benefit may require balancing multiple parameters such as energy density and irradiation time [17].
BMP-2 is a growth factor involved in skeletal development and bone repair belonging to the transforming growth factor-β (TGF- β) superfamily [19-21]. Mesenchymal cells of the osteoblast lineage differentiate through a series of progenitor stages to form functional, secretory osteoblasts [20,21]. In this process, BMP-2 acts as a chemoattractant for osteoprogenitor cells and drives differentiation of preosteoblasts by binding cell surface serine/threonine kinase receptors [22,23]. Mature osteoblasts assume a cuboidal shape, become polarized with distinct apical and basolateral surfaces, attach via noncollagen proteins such as osteopontin, and lay down organic matrix rich in type I collagen [24-26].
Nd:YAG laser energy may stimulate BMP-2 expression/secretion and enhance bone regeneration through multiple mechanisms [27,28]. In equine mesenchymal stem cell cultures, Nd:YAG laser stimulation significantly increased interleukin (IL)-10 and vascular endothelial growth factor (VEGF) without increasing cell proliferation [29]. In a rat femur model, Q-switched Nd:YAG laser stimulation increased osteoblast activity and decreased osteoclast number, resulting in significantly greater bone volume, trabecular thickness, and bone mineral density compared with controls [30]. In critical sized rat and rabbit calvarial defect models, Nd:YAG laser stimulation has accelerated bone formation compared with controls [31]. Nd:YAG laser effects on BMP-2 secretion may partially account for this observation. In preosteoblasts (MC3T3-E1 cells), Nd:YAG laser energy has increased BMP-2 gene expression and protein levels [11]. Laser stimulation alone or exposure to exogenous BMP-2 (100 ng/ml) produced comparable expression of the upstream regulator cbfa1 in MC3T3-E1 cells, while combination of Nd:YAG laser exposure and exogenous BMP-2 produced synergistic increases in ALP activity and expression of genes involved in bone repair such as IGF-1 and cbfa1 [11]. Nd:YAG laser exposure may also promote differentiation of osteoblast-like (MG-63) cells, as assessed by cell morphological changes observed in scanning electron microscopy and osteocalcin gene expression [17].
Contrary to findings of Kim et al.,[11]. consistent increases in BMP-2 secretion in laser-exposed cell cultures were not observed in the current investigation. Differences in target cell types and laser irradiation parameters may account for these discordant observations. The MC3T3-E1 cells utilized in the former study are osteoblast precursors, whereas MG-63 cells, considered “osteoblast-like,” derive from osteosarcoma [28]. Normal human osteoblasts are not terminally differentiated but are post-mitotic [28], and one of the important physiologic effects of BMP-2 is to induce differentiation of preosteoblasts to produce functional osteoblasts [1-3]. Thus, MG-63 and MC3T3-E1 cells may respond differently to exogenous BMP-2 or Nd:YAG laser stimulation. Additionally, BMP-2 expression/secretion may represent another outcome exhibiting a biphasic response. The exposure levels applied herein may have exceeded the optimal dose for the outcome of interest. Moreover, the irradiation parameters used in the current study may have induced photothermal in addition to photochemical and photophysical effects.
Conclusion
In MG-63 osteoblast-like cells, statistically significant increases in cell proliferation were observed 24 and 72 hours following Nd:YAG laser stimulation. However, Nd:YAG laser irradiation did not consistently influence BMP-2 secretion under the described experimental conditions. Cellular responses to Nd:YAG laser energy may be exquisitely dependent upon irradiation parameters, and overexposure may produce inhibitory effects.
Additionally, the observed effects of Nd:YAG laser irradiation may vary considerably depending upon the target cell type.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy of the United States Government, the Department of Defense, the Defense Health Agency, or Uniformed Services University of the Health Sciences.
Conflict of interest statement
The authors report no financial, economic, or professional interests that may have influenced the design, execution, or presentation of this work.
Author contribution statement
Conceptualization: Daniel DiPirro, Daniel Boudreaux, Thomas Johnson
Investigation: Daniel DiPirro, Daniel Boudreaux
Methodology: Daniel Boudreaux
Formal analysis: Brittany Ange, Thomas Johnson
Writing–original draft: Daniel DiPirro, Thomas Johnson, Brittany Ange
Writing–review & editing: Daniel Boudreaux, Thomas Johnson
References
- Urist MR. Bone: Formation by autoinduction. Science. 1965; 150:893–899. [PubMed.]
- Wozney JM., Rosen V., Celeste AJ., et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988; 242:1528–1534. [PubMed.]
- Wozney JM. Overview of bone morphogenetic proteins. Spine. 2002; 27:S2–S8. [PubMed.]
- Wikesjö UM., Qahash M., Polimeni G., et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008; 35:1001–1010. [PubMed.]
- Thoma DS., Yoon SR., Cha JK., et al. Sinus floor elevation using implants coated with recombinant human bone morphogenetic protein-2: micro-computed tomographic and histomorphometric analyses. Clin Oral Investig. 2018; 22:829–837. [PubMed.]
- Johnson TM., Jusino MA. Management of an immediate implant horizontal defect using freeze‐dried bone allograft and a neodymium: yttrium‐aluminum‐garnet laser. Clin Adv Periodontics. 2017;7:175–181. [PubMed.]
- Choi AY., Reddy CM., McGary RT., et al. Adjunctive Nd: YAG laser irradiation for ridge preservation and immediate implant procedures: a consecutive case series. Clin Adv Periodontics. 2019; 9:125–134. [PubMed.]
- Cheng AW., Berridge JP., McGary RT., Erley KJ., Johnson TM. The Extraction Socket Management Continuum: A Hierarchical Approach to Dental Implant Site Development. Clin Adv Periodontics. 2019; 9:91–104. [PubMed.]
- Mills MP., Rosen PS., Chambrone L., et al. American Academy of Periodontology best evidence consensus statement on the efficacy of laser therapy used alone or as an adjunct to non–surgical and surgical treatment of periodontitis and peri–implant diseases. J Periodontol. 2018; 89:737–742. [PubMed.]
- Chambrone L., Ramos UD., Reynolds MA. Infrared lasers for the treatment of moderate to severe periodontitis: An American Academy of Periodontology best evidence review. J Periodontol. 2018; 89:743–765. [PubMed.]
- Kim IS., Cho TH., Kim K., Weber FE., Hwang SJ. High power‐pulsed Nd: YAG laser as a new stimulus to induce BMP‐2 expression in MC3T3‐E1 osteoblasts. Lasers Surg Med. 2010; 42:510–518. [PubMed.]
- Pyo SJ., Song WW., Kim IR., et al. Low–level laser therapy induces the expressions of BMP–2, osteocalcin, and TGF–β1 in hypoxic–cultured human osteoblasts. Lasers Med Sci. 2013; 28:543–550. [RePubMedf.]
- Fujimoto K., Kiyosaki T., Mitsui N., et al. Low‐intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3‐E1 cells. Lasers Surg Med. 2010; 42:519–526. [PubMed.]
- Ueda Y., Shimizu N. Effects of pulse frequency of low–level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg. 2003; 21:271–277. [PubMed.]
- Karoussis IK., Kyriakidou K., Psarros C., Lang NP., Vrotsos IA. Nd:YAG laser radiation (1.064 nm) accelerates differentiation of osteoblasts to osteocytes on smooth and rough titanium surfaces in vitro. Clin Oral Implants Res. 2017; 28:785–790. [PubMed.]
- Arisu HD., Türköz E., Bala O. Effects of Nd:YAG laser irradiation on osteoblast cell cultures. Lasers Med Sci. 2006; 21:175–180. [PubMed.]
- Aleksic V., Aoki A., Iwasaki K., Takasaki AA., et al. Low–level Er: YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med Sci. 2010; 25:559–569. [PubMed.]
- Altan BA., Sokucu O., Ozkut MM., Inan S. Metrical and histological investigation of the effects of low–level laser therapy on orthodontic tooth movement. Lasers Med Sci. 2012; 27:131–140. [PubMed.]
- Chen DI., Zhao M., Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004; 22:233–241. [PubMed.]
- Duplomb L., Dagouassat M., Jourdon P., Heymann D. Concise review: embryonic stem cells: a new tool to study osteoblast and osteoclast differentiation. Stem Cells. 2007; 25:544–552. [PubMed.]
- Rutkovskiy A., Stensløkken KO., Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016; 22:95–106. [PubMed.]
- Sieber C., Kopf J., Hiepen C., Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009; 20:343–355. [PubMed.]
- Harada SI., Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003; 423:349–355. [PubMed.]
- Liu F., Malaval L., Aubin JE. The mature osteoblast phenotype is characterized by extensive plasticity. Exp Cell Res. 1997; 232:97–105. [PubMed.]
- Sodek J., Mckee MD. Molecular and cellular biology of alveolar bone. Periodontol. 2000 2000; 24:99–126. [PubMed.]
- Sodek J., Ganss B., McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. [PubMed.]
- Hosseinpour S., Fekrazad R., Arany PR., Ye Q. Molecular impacts of photobiomodulation on bone regeneration: a systematic review. Prog in Biophys Mol Biol. 2019; 149:147–159. [PubMed.]
- Deana AM., de Souza AM., Teixeira VP., Mesquita–Ferrari RA.,et al. The impact of photobiomodulation on osteoblast–like cell: a review. Lasers Med Sci. 2018; 33:1147–1158. [PubMed.]
- Peat FJ., Colbath AC., Bentsen LM., Goodrich LR., King MR. In vitro effects of high–intensity laser photobiomodulation on equine bone marrow–derived mesenchymal stem cell viability and cytokine expression. Photomed Laser Surg. 2018; 36:83e91. [PubMed.]
- Ninomiya T., Hosoya A., Nakamura H., Sano K., Nishisaka T.,et al. Increase of bone volume by a nanosecond pulsed laser irradiation is caused by a decreased osteoclast number and an activated osteoblasts. Bone. 2007; 40:140–148. [PubMed.]
- Kim K., Kim IS., Cho TH., Seo YK., Hwang SJ. High–intensity Nd:YAG laser accelerates bone regeneration in calvarial defect models. J Tissue Eng Regen Med. 2015; 9:943–951. [PubMed.]