>Corresponding Author : Sahel Imane
>Article Type : Case Report
>Volume : 5 | Issue : 11
>Received Date : 09 October, 2025
>Accepted Date : 20 October, 2025
>Published Date : 27 October, 2025
>DOI : https://doi.org/10.54289/JCRMH2500155
>Citation : Imane S, Ezahraa TF, Amina G, Boufettal H, Mahdaoui S, et al. (2025) Endometriosis of the Abdominal Wall: Three Cases Report. J Case Rep Med Hist 5(11): doi https://doi.org/10.54289/JCRMH2500155
>Copyright : © 2025 Imane S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Resident Physician, Department of Gynecology and Obstetrics at the Ibn Rochd University Hospital, Casablanca, Morocco
2Professor in the Department of Gynecology and Obstetrics at the Ibn Rochd University of Hospital in Casablanca Morocco
*Corresponding author: Sahel Imane, Resident Physician, Department of Gynecology and Obstetrics, at Ibno Rochd University Hospital, Casablanca, Morocco
Abstract
Introduction and Importance: Endometriosis of the abdominal wall is a rare disease that usually develops on a caesarean scar. Although frequently observed in the cutaneous and subcutaneous adipose tissue in front of the caesarean scar, its intramuscular localisation is possible but remains rare.
Case Presentation: We report three cases of parietal endometriosis occurring on a Pfannenstiel scar after caesarean section, their surgical management and evolution, in the department of gynecological-mammary surgery of CHU Ibn Rochd Casablanca Hospital.
Clinical Discussion: Endometriosis is defined as the ectopic implantation of the functional endometrial epithelium outside the uterine cavity. The pathophysiology of endometriosis is still poorly understood. The interest of the scanner lies in the study of the deep invasion of the lesions. MRI is the examination of choice. The reference treatment for parietal endometriosis is wide surgical exeresis from the outset, even if it means performing a parietoplasty to fill in the aponeurotic dehiscence.
Conclusion: Parietal endometriomas are rare. Location on a caesarean scar is the most common. Diagnosis can be difficult. Surgery must be extensive to avoid recurrence. The diagnosis is only confirmed by anatomopathological study. No efficacy of intraoperative preventive measures has yet been demonstrated.
Keywords: Abdominal wall, Endometriosis, Surgery, Clinical case
Abbreviations: CT: Computed Tomography
Introduction
Endometriosis is defined as the abnormal implantation of functional endometrial epithelium with stroma outside the uterine cavity. It affects women during their genital period with a prevalence of 10%. Its main locations are endopelvic, mainly involving the internal genitalia (ovaries, fallopian tubes, myometrium), the utero sacral ligaments, the broad ligament and the bladder. The location in the extra genital organs is less described. Parietal endometriosis is a rare clinical entity; its occurrence in postoperative scars, particularly Pfannenstiel scars, is even less rare (0.03 to 0.4% of all endometriosis) but is the most frequent parietal location [1]. It is suspected on the basis of a number of clinical and radiological arguments, but is confirmed histologically.
Parietal endometriosis reveals a surgical treatment. We report three observations of parietal endometriosis occurring on Pfannenstiel scars collected in the gynaecological and breast surgery department of Ibn Rochd Hospital in Casablanca. We ensure that the work was reported in accordance with the SCARE 2020 criteria [2].
Patients and Observations
Observation 1
Patient information: a 31 year old female patient, with no previous history; G3P3, she had three caesarean deliveries, the last one in 2020, not known to be a genito- pelvic endometriosis carrier; who consulted for a painful swelling at the Pfannenstiel scar, evolving for 6 months (i.e. a delay of 10 months after the delivery) The history found a catamenial character of the symptomatology: exacerbation of pain during menstruation, without any notion of dysmenorrhea, dysuria or dyspareunia. In addition, the patient reported a cyclical increase in the volume of the mass during menstruation.
Clinical examination findings: The clinical examination revealed an oval mass with a long axis of 3 cm at the left angle of the Pfannenstiel scar, poorly limited, not very mobile, and tender. Pelvic touch was unremarkable.
Diagnostic approach: Soft tissue ultrasound found an 18 mm heterogeneous hypoechoic lesion, located opposite the left side of the pelvic scar. Abdominopelvic computed tomography (CT) found a 3cm oblong mass, adherent to the left rectus muscle and its fascia, hypodense and poorly limited (Figure 1A, Figure 1B). Biopsies were not taken. The diagnosis of endometriosis was strongly suggested by this set of anamnestic, clinical and para-clinical arguments and the decision was made to operate on the patient.
Surgical treatment: the patient was operated on by a revision on the old scar by an experienced surgeon, exploration had found a 3 cm nodule embedded in the rectus muscle, she had a large exeresis of the parietal mass taking away the part of the rectus muscle and its aponeurosis opposite the lesion, with a repair of the parietal dehiscence by a simple perineorrhaphy
Final diagnosis: the anatomopathological study confirmed the diagnosis of parietal endometriosis by showing a focus of endometriosis in the abdominal wall.
Follow-up and evolution: The immediate postoperative follow-up was simple. Follow-up after 6 months did not detect any recurrence.
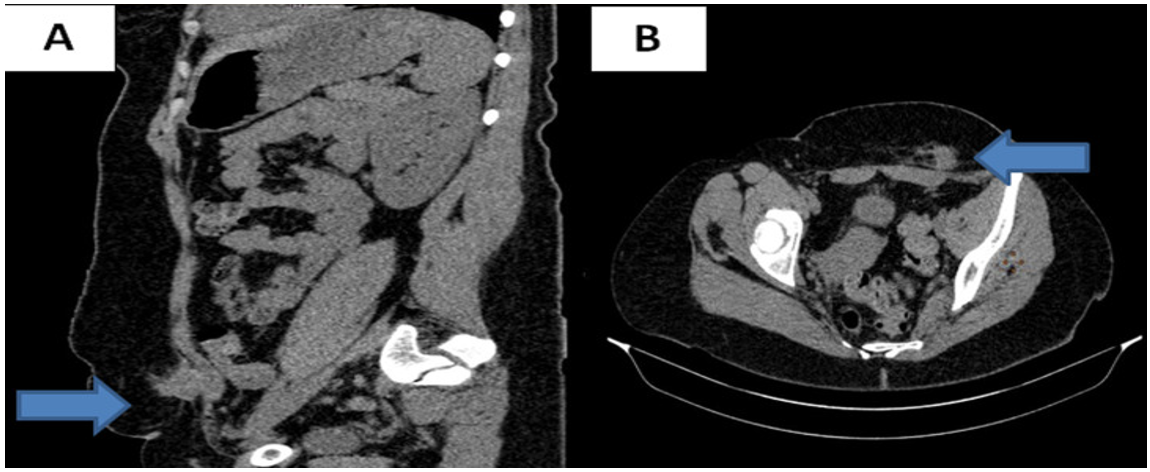
Figure 1: Abdominopelvic CT scan: parietal lesion in the rectus muscle: (A) sagittal section, (B) axial section
Observation 2
Patient information: 32 year old female patient with a history of Mac Burney appendectomy at the age of 15, G2P2; 2 caesarean deliveries in 2016 and 2019, who presented 2 years prior to admission with a painful nodule at the left angle of the Pfannenstiel scar: She reported a chronic pain background with a cyclic exacerbation concomitant with menstruation, otherwise she did not report similar pain in relation to Mac Burney scar. Questioning revealed dysmenorrhoea since the second delivery. For a few months prior to admission the patient reported a change in skin colouring in relation to the nodule, becoming bluish during menstruation.
Clinical examination findings: A 2cm nodule was found in the left corner of the Pfannenstiel scar, the Mac Burney scar was solid and without abnormalities.
Diagnostic approach: abdominal CT scan found two nodules of 3x2 cm and 1x2 cm in the rectus muscles on either side of the Pfannenstiel scar, of the same density before and after injection of contrast medium as the uterus, suggesting endometriosis lesions. Pelvic MRI was performed to better identify the lesions and to look for deep pelvic endometriosis lesions warranting possible medical treatment. Pelvic MRI showed multiple endometriotic nodules over the entire caesarean section scar, including one measuring 17x19 mm embedded in the subcutaneous fat and in the thickness of the right and left rectus muscles (Figure 2). In addition, there were no lesions in favour of deep pelvic endometriosis.
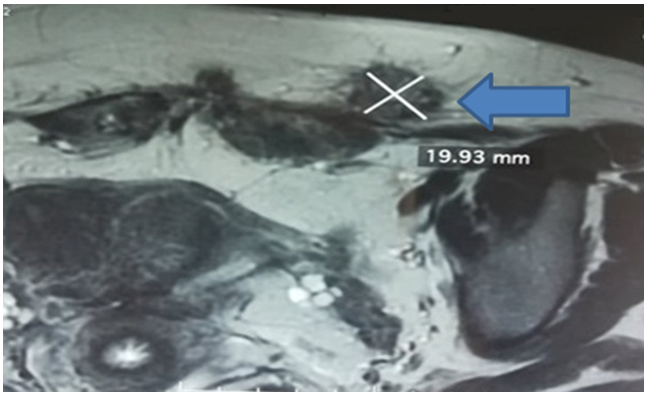
Figure 2: Coronal section of a T1-weighted pelvic MRI fat saturation sequence (FATSAT); left paramedian nodule located on the caesarean scar measuring 17x19 mm
Surgical treatment: the patient was operated, by an experienced surgeon, on using the old Pfannenstiel scar, a nodule was found with connections to the aponeurosis of the left rectus muscle, and another sub-aponeurotic nodule with connections to the rectus muscles on the right and extending to the pubis (Figure 3). A wide muscle and aponeurotic excision involving the nodules was performed through healthy tissue, resulting in a monobloc resection. Given the extent of the loss of substance on the abdominal wall, exposing the risk of hernia, a parietoplasty was performed by interposing a polypropylene plate in the prefascial retro-muscular region (Figure 4).
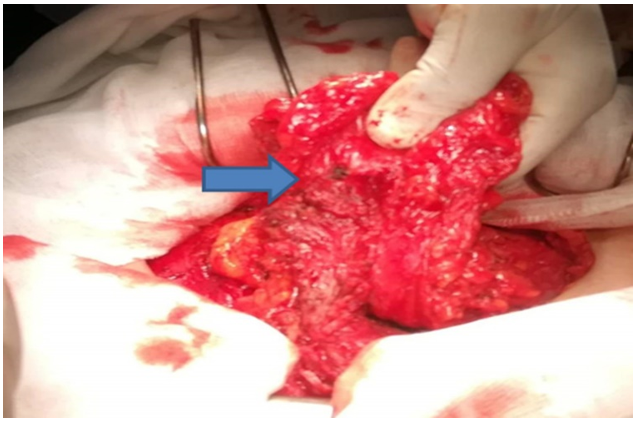
Figure 3: Intraoperative view, endometriosis nodule on the rectus muscle
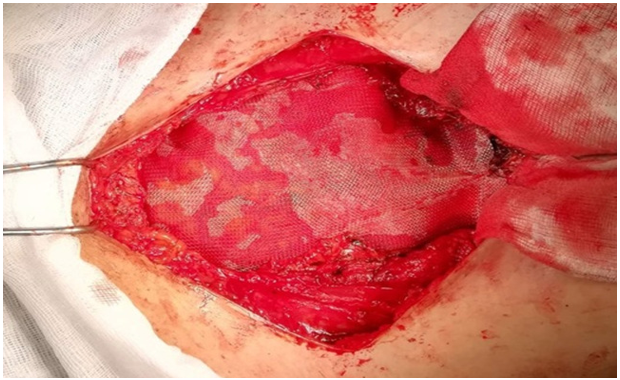
Figure 4: Intraoperative view, parietal repair with a retro-muscular prefacial plate
Final diagnosis: gross pathological examination revealed 2 fatty pieces with numerous haemorrhagic foci on section. The histological study confirmed the diagnosis of parietal endometriosis with the presence of glands bordered by a pseudo-stratified epithelium with the presence of cytogenic chorion (Figure 5A, Figure 5B).
Follow-up and evolution: the immediate postoperative course was simple. Follow-up after 6 months did not detect any recurrence.
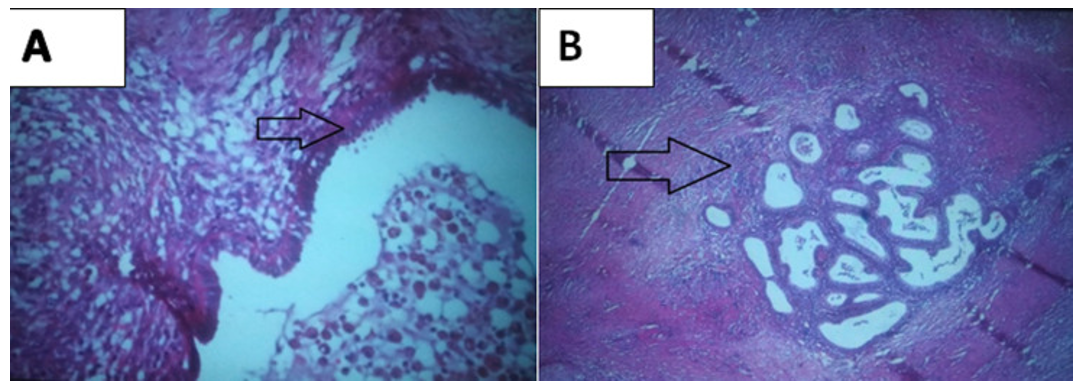
Figure 5: Histological aspect (HEx40), A) presence of glands bordered by a pseudo stratified epithelium with presence of cytogenic chorion; B) focus of endometriosis within the abdominal wall
Observation 3
Patient information: The patient was 31 years old, with no previous history of malformations; G1P4, she had a caesarean delivery for quadruplets 4 years ago; she consulted for a painful parietal mass in front of the pfannual scar. The onset of the symptomatology was several months ago, with the progressive development of a painful Pfannestiel scar mass, with periodic cyclic pain. The patient was treated with a GnRH analogue for 6 months with 1 injection every 3 months as a result of suspected parietal endometriosis without significant improvement.
Clinical examination data: the clinical examination found a mass of 8 cm long, hard axis opposite the pfannestiel scar, painful to palpation, fixed in relation to the superficial and deep plane. The gynaecological examination was unremarkable.
Diagnostic approach: Parietal and pelvic ultrasound showed a double echogenic parietal mass in the rectus abdominis measuring 50 and 30 mm respectively. Pelvic MRI showed two infiltrating masses in the abdominal wall involving the rectus abdominis muscles in T1 hyposignal, with moderate T2 heterogeneous signal taking up Gadolinium, with the presence presence of spots in T1 hypesignal FATSAT in relation to haemorrhagic stigmata, these two masses measuring 57x28x52 mm on the left and 51x20x26 mm on the right.
Both lesions extend beyond the muscle and infiltrate the subcutaneous tissue. The left mass reaches the anterior wall of the bladder which is thickened at this level. No other parietal or pelvic locations. Appearance in favour of pelvic and parietal endometriosis
Surgical treatment: The treatment consisted of a wide exeresis, by an experienced surgeon, of the lesions with re-dissection of the pfannestiel scar taking away part of the skin, dissection of the wall in relation to the fascia of the rectus abdominis muscle, carrying out an en bloc exeresis of the masses taking away part of the fascia and the rectus abdominis muscle and closure carried out plane by plane without the use of a plate (Figure 6-7).
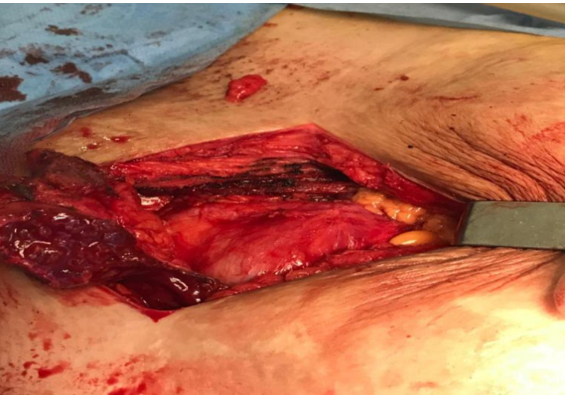
Figure 6: Removal of the aponeurosis and rectus muscle carrying the masses to the bladder plane
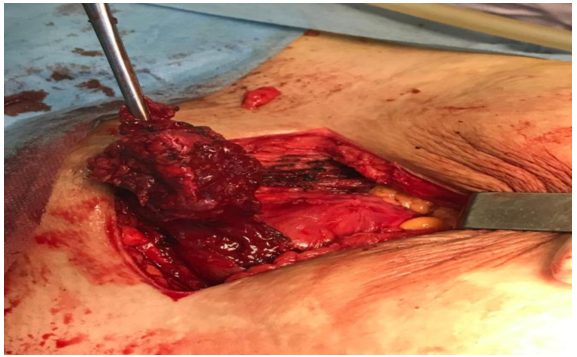
Figure 7: Wide lumpectomy after opening of the rectus fascia
Final diagnosis: The anatomopathological study confirmed the endometriotic nature of the mass, in favour of the diagnosis of parietal endometriosis.
Follow-up and evolution: the immediate postoperative follow-up was simple. Follow-up after 6 months did not detect any recurrence.
Discussion
Endometriosis is defined as ectopic implantation of the functional endometrial epithelium outside the uterine cavity [1]. It is a chronic condition that is often diagnosed late in the course of the disease due to a lack of awareness of the condition. It mainly affects women of childbearing age, with a peak around the age of 40 [1]. The main locations are in the pelvis. Parietal location is rare. The location on gynaecological or obstetric surgery scars, which is of interest, is even rarer [1,3]. The pathophysiology of endometriosis is still poorly elucidated and uncertain, but several theories have been put forward, including [1,3].
1) Implantation theory: described in 1927 by Sampson: endometriotic cells originating from the reflux of menstrual blood through the fallopian tubes into the peritoneal cavity implant ectopically. In the presence of a malfunctioning sewage system or due to the abundance of reflux. The cells infiltrate the mesothelium of the vessels of the invaded tissues generating angiogenesis.
2) The metaplastic theory: according to this theory, the cells of the coelomic epithelium (tissue of embryological origin present in the peritoneum, pleura and pericardium) undergo metaplasia into endometrial cells, and this theory could explain the cases of pleural localisations and the cases observed in men under osteogenic treatment.
3) The metastatic theory: this theory states that the dissemination of endometrial cells is possible by lymphatic and vascular routes.
In our context: parietal endometriosis can be explained by the implantation theory because it is an iatrogenic and involuntary dissemination of endometrial cells during abdominopelvic surgery. Several risk factors have been incriminated in the genesis of endometriosis, including genital activity and any prolonged exposure to oestrogens (early puberty, late menopause) but also prolonged intervals between pregnancies. Menstrual disorders have also been incriminated: endometriotic women usually have short cycles with a heavy and prolonged flow, which could correspond to exposure to heavy and more frequent menstrual reflux. Congenital anomalies of the genital tract are also considered a risk factor. Indeed, any obstacle to the normal flow of menstrual fluid potentially favours reflux. For our three patients, not known to be carriers of genitopelvic endometriosis, the average age of diagnosis was 31.3 years. The age of menarche was at 14, 11 and 12 years respectively. Cycles were regular 27+/- 1 day with no flow abnormalities in all three patients. Clinically: The symptomatology of parietal endometriosis is essentially a painful mass with a catamenial character. This cyclical nature of the pain is an important element of orientation but not indispensable to evoke the diagnosis. These signs may appear in the weeks or even years following the operation. In our patients, The interval between the operation and the onset of symptoms was 10 months for the first patient, 12 months for the second, and 13 months for the third [1,3]. The lesions on the surgical scars, which are usually located in front of the Pfannenstiel scar, are usually painful masses averaging 2 cm in size. A cyclical change in the behaviour of very superficial lesions, such as bleeding and fistulation to the skin, has also been described [1].
In one of the patients, a change in skin colouration in front of the mass during menstruation was noted. The parietal location in our patients was on the Pfannenstiel scar. It should be noted that the Mac Burney scar of the 2nd patient was free of endometrioma. The ultrasound and CT images of scar endometriosis are not very specific. They are either liquid collections or tissue-like. The cystic/tissue appearance of the lesion depends on the hormonal impregnation according to the cycle. The fluid component was found on ultrasound in the first patient [4]. The interest of CT lies in the study of the deep invasion of the lesions. MRI is the examination of choice for deep endometriotic lesions indicating medical treatment associated with excisional surgery [1,4]. In one of our patients, the diagnosis was suggested by CT and confirmed by MRI. The serum CA125 level may be increased in correlation with the epithelial proliferation of the endometriotic lesions. In our patients tumour markers were not requested. Confirmation of the diagnosis is histological, showing the presence of ectopic endometrial glands with glandular tubes, cytogenic chorion and muscle fibres on the surgical specimens.
The standard treatment for parietal endometriosis is wide surgical excision from the outset, even if it means performing a parietoplasty to fill in the fascial dehiscence [3,5]. Although no benefit has been demonstrated, it is advisable in the case of laparotomy to wash the scar abundantly and to change gloves before closing it. In case of laparoscopy, to prevent endometriotic grafting on the trocar holes, it is good surgical practice to remove the operative parts in a protective bag systemically [1,4,5].
Conclusion
Parietal endometriomas are rare. The location on a caesarean scar is the most frequent. The diagnosis can be difficult but should be made in the presence of a painful and cyclic parietal tumour on a gynaecological or obstetric scar. Radiological examinations are used to characterise the lesion (number, location, size, depth) and to look for possible signs of deep disease indicating medical treatment after removal of superficial lesions. Surgery must be extensive to avoid recurrence. The diagnosis is only confirmed by anatomopathological study. Despite the fact that parietal endometriosis is thought to be caused by accidental iatrogenic implantation during intraoperative wound manipulation, the effectiveness of intraoperative preventive measures has not yet been demonstrated.
Conflicts of interest: The authors declare that they have no conflict of interest.
Sources of funding: We declare there was no sources of funding
Ethical Approval: The authors institute provided ethical approval for this case study.
Consent: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in Chief of this journal on request.
References
- Patil N., Kumar V., Gupta A. Scar endometriosis: a sequel of caesarean section. J Clin Diagn Res. 2014;8(4):FD09–10. [PubMed.]
- Agha RA., Franchi T., Sohrabi C., Mathew G., The SCARE Group. The SCARE 2020 Guideline: Updating Consensus Surgical Case Report (SCARE) Guidelines. International Journal of Surgery. 2020;84:226–230.2. [PubMed.]
- Emre A., Akbulut S., Yilmaz M., Bozdag Z. Laparoscopic trocar port site endometriosis: a case report and brief literature review. Int Surg. 2012;(2):135–9. [Ref.]
- Sinha R., Kumar M., Matah M. Abdominal scar endometriosis after Cesarean section: a rare entity. Australas =Med J. 2011;4(1):60–2. [PubMed.]
- Cöl C., Yilmaz EE. Cesarean scar endometrioma: case series. World J Clin Cases. 2014;16;2(5):133–6. [PubMed.]