>Corresponding Author : Benhaddouga Khadija
>Article Type : Case Report
>Volume : 5 | Issue : 10
>Received Date : 11 July, 2025
>Accepted Date : 21 July, 2025
>Published Date : 25 August, 2025
>DOI : https://doi.org/10.54289/JCRMH2500149
>Citation : Benhaddouga K, Hlaibi O, Abdouni A, Meryem Z, Boufettal H, et al. (2025) About Two Cases of Uterine Smooth Muscle Tumor of Uncertain Malignant Potential. J Case Rep Med Hist 5(10): doi https://doi.org/10.54289/JCRMH2500149
>Copyright : © 2025 Khadija B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Department of Gynecology and Obstetrics, University Hospital Center Ibn Rochd, Casablanca 20100, Morocco
2Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Casablanca, Morocco
*Corresponding author: Benhaddouga Khadija, Department of Gynecology and Obstetrics, University Hospital Center Ibn Rochd, Casablanca 20100, Morocco and Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Casablanca, Morocco
Summary
Uterine smooth muscle tumor of uncertain malignant potential (STUMP) is a rare tumor. They are classified as benign leiomyoma and malignant leiomyosarcoma regarding presence of tumor cell necrosis, cytological atypia and mitotic activity. STUMP is regarded in World Health Organization (WHO) classification as smooth muscle tumors between benign and malignant criteria.
STUMP is seen mostly in age of mid-forties, who has been operated with preoperative leiomyoma diagnosis. Risk factors and prognosis are not fully understood, but in long-term follow-up, there is a potential of recurrence or metastasis.
Keywords: Uterine Leiomyoma; Atypical Leiomyoma; Uterinesmooth Muscle Tumor of Uncertain Malignant Potential (STUMP); Smooth Muscle Tumor of Low Malignant Potential; Leiomyosarcoma; P16; P53
Abbreviations: STUMP: Smooth Muscle Tumor of Uncertain Malignant Potential, WHO: World Health Organization, RMN: Magnetic Resonance, PET: Positron Emission Tomography, FDG: Fluorodeoxyglucose, CT: Computed Tomography
Introduction
According to the World Health Organization (2003), uterine smooth muscle tumor of uncertain malignant potential (STUMP) is a borderline tumor between benign leiomyoma and malignant leiomyosarcoma ;It is difficult for an even skilled pathologist to diagnose STUMP by identifying detailed markers such as cytologic atypia, mitotic activity, or tumor cell necrosis that distinguish leiomyoma from leiomyosarcoma ;The clinical features, prognostic factors, and optimal management of STUMP are poorly understood because of limited data associated with its rarity.
Patients with STUMP may have symptoms such as abnormal uterine bleeding, pelvic pain, and lower abdominal pressure consistent with benign uterine myoma, although there may be differences depending on the size of uterine mass. Because STUMP and benign uterine myoma are not significantly different in terms of preoperative radiologic imaging and laboratory tests, it is difficult to distinguish between these tumors prior to pathological confirmation at surgery. Conventional surgical management of STUMP includes myomectomy or hysterectomy. Myomectomy in limited cases may be considered in women who wish to preserve fertility. Here, we report two cases of STUMP presenting as a giant uterine mass that was suspected to be a benign uterine myoma preoperatively.
Case Presentation
45 years old, single, nulligest, still in a period of reproductive activity, admitted for chronic pelvic pain evolving 6 months ago in a context of conservation of general condition; in whom the examination found an abdominal-pelvic mass reaching the umbilicus measuring 16 cm (fig 1).
Fig 1: Abdominal-pelvic mass arriving at the umbilicus measuring 16 cm
Pelvic ultrasound showed the presence of a bully uterine tissue mass, roughly oval, with lobulated contours, discreetly vascularized on color Doppler measuring 15x9cm.
Abdomino-pelvic CT objectified the presence of an intra-uterine tissue mass lateralized on the left heterogeneously enhanced after injection of PDC, fairly well limited, seat of the area of necrosis, measuring 127x80x165cm. Associated with left external iliac adenopathy of 8 mm Probably related to uterine myoma.
The patient underwent a myomectomy (fig4).
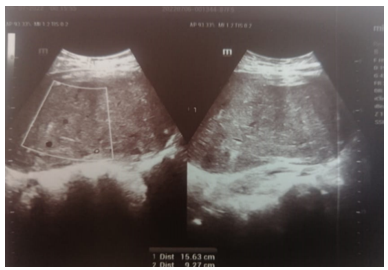
Fig 2: Echographic aspect tissue mass of heterogeneous uterine origin measuring 15 cm
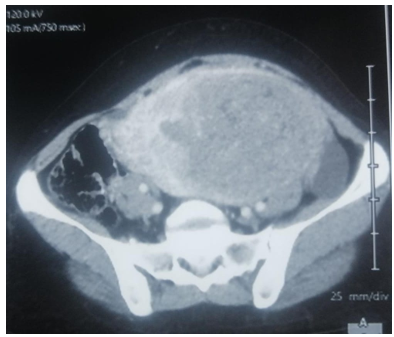
Fig 3: CT appearance of a tissue mass of hetero-dense uterine origin poorly limited in places measuring 15 cm
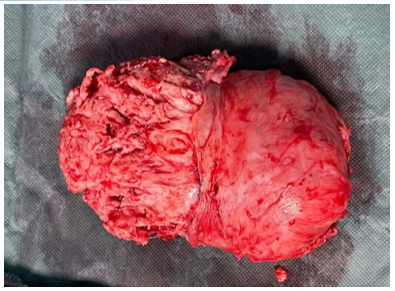
Fig 4: Myomectomy Surgical Specimen
Histopathological examination revealed fasciculated tumour proliferation, dissociated by hyaline fibrous remodelling. The tumour was composed of smooth muscle cells with spindle-shaped nuclei showing moderate to severe cytonuclear atypia. cytoplasm was abundant and poorly limited. Mitotic count showed 9 mitoses/10 CFG with no necrosis, suggesting a smooth muscle tumor of uncertain malignancy potential.
Case report 2
45-year-old patient, still in a period of reproductive activity mother of a live child, who has been followed for retroviral infection since 2009 and underwent polymyomectomy in 2018.
Consulted for chronic pelvic pain associated with low-grade menometrorrhagia, in whom clinical examination revealed an enlarged uterus reaching the umbilicus.
Pelvic ultrasound revealed an enlarged uterus measuring 145x84x80mm with bumpy contours and heterogeneous echostructure with the presence of multiple heterogeneous hypoechoic myomas of variable size, the largest of which was corporal-fundial measuring 62x59x48mm classified FIGO 2-5 and a submucosal myoma measuring 27x23x23m classified FIGO 1.
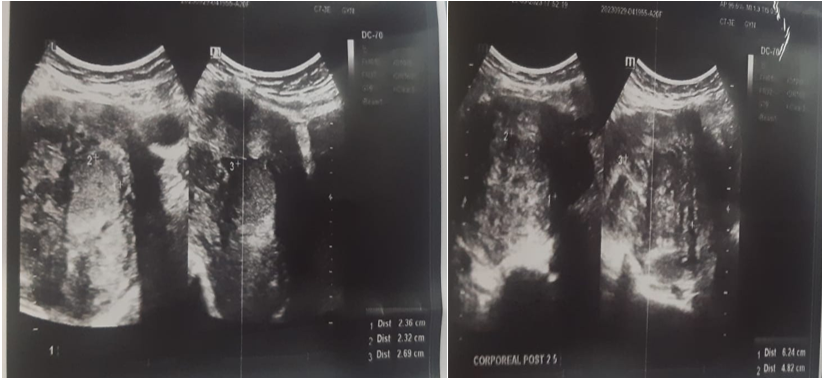
Fig 5: Pelvic ultrasound revealed multiple heterogeneous hypoechoic myomas.
Pelvic MRI revealed a polymyomatous uterus, with a FIGO 2-5 resorbed fibroid occupying almost the entire uterine cavity, and a small FIGO 6 subserous myoma measuring 28x21 mm.
A polymyomectomy was performed, with the following anatomopathological findings.
Large myoma: à large, partially encapsulated tumour measuring 12 cm in diameter, with a friable, greyish white apparence, corresponding to a smooth, cellular muscular tumour of uncertain malignancy (stump), associated with peripheral, florid adenomyosis.
Small myomas: two small uterine leiomyomas, reshaped and benign.
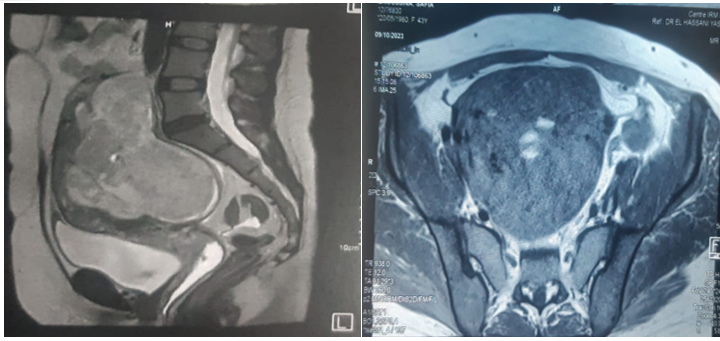
Fig 6: MRI suggestive of polymyomatous uterus
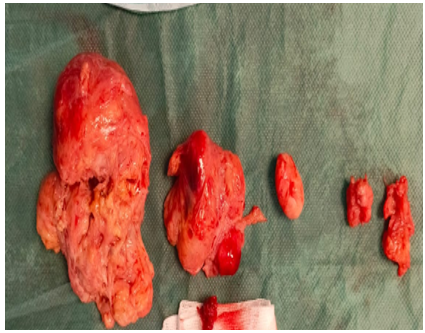
Fig 7: Polymyomectomy surgical parts
Discussion
To the best of our knowledge, the term ‘‘smooth muscle tumor of uncertain malignant potential’’ was first used in the literature by Kempson in 1973. The criteria for these tumors have evolved over the ensuing 35 years as our knowledge of the behavior of uterine SMTs with varying combinations of atypical features has accrued.
STUMP tumors are rare and are commonly diagnosed by histopatholological evaluation after surgical excision of the tumor.
Because of this, only retrospective case studies have been reported in the previous literature [1-3]. Consequently, the frequency of STUMP is difficult to determine. Since there is a risk of recurrence, studies have been conducted to investigate the prognostic factors [2,4]. But it was stated that race/ethnicity, smoking and type of surgery are not indicative of recurrence [2]. In addition, optimal follow-up recommendations were not determined in studies with limited number of patients [2,4].
Magnetic resonance (RMN) and pelvic ultrasounds are not reliable methods to pre-operatively differentiate benign from malignant tumors [5] or characterize smooth muscle uterine tumors [6]. The usefulness of 18fluorodeoxyglucose [FDG] positron emission tomography (PET) and computed tomography (CT) scans is debated, and few studies have been published [7].
The median age at diagnosis was 43 years, in agreement with data already publishedin the literature (mean/median age of patients: 41/48 years) [6]. The mean diameter oflesions was 8.0 cm, and most STUMPs showed intramural localization, a low or moderatenumber of mitoses, mild or moderate atypia, and absent necrosis, according to Stanfordcriteria [8].
The prognosis of STUMP is better than that of leiomyosarcoma (LMS) Peters et al. [9] studied 15 STUMP and 22 LMS and found 27% recurrence for STUMP against 69% for LMS with an overall five-year survival of 92% against 40%. In the context of LMS, local recurrences or at a distance are generally observed within two years of following the diagnosis. On the other hand, in the STUMP this recurrence may be much later. Beretta et al. [10] reports a recurrence in the form of pulmonary metastasis nine years after a diagnosis of STUMP. Finally, the team of MD Anderson reports his experience of 41 patients with the diagnosis of STUMP treated by myomectomy or hysterectomy, without additional treatment, and a follow-up average of 45 months [11]. Three recurrences (7.3%) are reported between 16 and 63 months, at the pelvic or pulmonary level, under a mode of STUMP (two cases) or leiomyosarcoma (one case), without any deaths [11].
Immunohistochemical overexpression of p16 was correlated with the prognosis of STUMP [8]. Indeed, this overexpression is found in 80% of leiomyosarcomas of diffusely, in 13% of leiomyomas focally, and in 66% of the STUMPs which presented an evolution pejorative [12]. In STUMPs and LMSs, this is a intense and diffuse nuclear and/or cytoplasmic staining.
However, basing the diagnosis solely on immunohistochemistry should be avoided. In fact, an overexpression of p16 and p53 has been described respectively 85% and 38% of atypical (or odd cell) leiomyomas [13].
Conclusion
STUMP is classified as an intermediate form, histopathologically so calling it benign or malignant for sure is not possible. Singulary, solidity, hyperechogenicity, heterogenecity and features of acoustic shadowing and margins can guide us to preoperative sonographic diagnosis. Recurrence/metastasis after many years from operation can be seen, and those patients should be followed long term.
References
- Bell SW., Kempson RL., Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18(6):535–58. [PubMed.]
- Saketh R Guntupalli., Pedro T Ramirez., Matthew L Anderson., Michael R Milam., et al. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009;113(3):324–6. [PubMed.]
- Ip PP., Cheung AN., Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33(7):992–1005. [PubMed.]
- Basaran D., Usubutun A., Salman MC., Narin MA., Boyraz G., Turkmen O., et al. The clinicopathological study of 21 cases with uterine smooth muscle tumors of uncertain malignant potential: centralized review can purify the diagnosis. Int J Gynecol Cancer. 2018;28(2):233–40. [Ref.]
- Yadav G., Rao M., Goyal SB., Singh P., Kathuria P., Gothwal M. Risk of incidental genital tract malignancies at the time of myomectomy and hysterectomy for benign conditions. Obstet. Gynecol Sci. 2020;64(2):209–215. [PubMed.]
- Gadducci A., Zannoni GF. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol. Oncol. 2019;154(3):631–637. [PubMed.]
- Ho K C., Dean Fang YH., Lin G., Ueng SH., Wu TI., Lai C H., et al. Presurgical Identification of Uterine Smooth Muscle Malignancies through the Characteristic FDG Uptake Pattern on PET Scans. Contrast. Media Mol. Imaging. 2018;7890241. [PubMed.]
- Bell S W., Kempson RL., Hendrickson MR. Problematic uterine smooth muscle neoplasms: A clinicopathologic study of 213 cases. Am. J. Surg. Pathol. 1994;18(6):535–558. [PubMed.]
- Peters 3rd WA., Howard DR., Andersen WA., Figge DC. Uterine smooth-muscle tumors of uncertain malignant potential. Obstet Gynecol. 1994;83(6):1015–20. [PubMed.]
- Berretta R., Rolla M., Merisio C., Giordano G., Nardelli GB. Uterine smooth muscle tumor of uncertain malignant potential: a three-case report. Int J Gynecol Cancer. 2008;18(5):1121–6. [PubMed.]
- Guntupalli SR., Ramirez PT., Anderson ML., Milam MR., Bodurka DC., Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009;113(3):324–6. [PubMed.]
- Atkins KA., Arronte N., Darus CJ., Rice LW. The use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32(1):98–102. [PubMed.]
- Sung CO., Ahn G., Song SY., Choi YL., Bae DS. Atypical leiomyomas of the uterus with long-term follow-up after myomectomy with immunohistochemical analysis for p16INK4A., p53, Ki-67, estrogen receptors., and progesterone receptors. Int J Gynecol Pathol. 2009;28(6):529–34. [PubMed.]