>Corresponding Author : Sanaa Benrahhal
>Article Type : Case Report
>Volume : 5 | Issue : 9
>Received Date : 24 May, 2025
>Accepted Date : 06 June, 2025
>Published Date : 14 August, 2025
>DOI : https://doi.org/10.54289/JCRMH2500146
>Citation : Benrahhal S, Elhachami F, Driss E, Jalal M, Lamrissi A, et al. (2025) About A Case: A Cardiac Acephalic Twin. J Case Rep Med Hist 5(9): doi https://doi.org/10.54289/JCRMH2500146
>Copyright : © 2025 Benrahhal S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
Centre Hospitalo Universitaire Elharouchi Casablanca Morocco, Gynecologic, Obstetric, Casablanca, Morocco Department of Maternity
*Corresponding author: Sanaa Benrahhal, Centre Hospitalo Universitaire Elharouchi Casablanca, Morocco
Abstract
Monochorionic twin pregnancies are characterized by the presence of vascular connections between the twins. These can be the cause of pathologies such as transfuser-transfused syndrome or TRAP syndrome, defined as the association of an acephalic acardiac twin and a healthy twin. This syndrome is very rare and is associated with a high mortality rate in the healthy twin due to anemia and heart failure. Prenatal diagnosis is possible, enabling appropriate monitoring and therapeutic measures to be taken to interrupt the vascular anastomoses between the twins, thereby achieving selective fœticide. Obstetric management varies from abstention to intervention, depending on the prognosis of the healthy twin, which is dominated by prematurity and heart failure. We report a case of an acephalic acardiac twin diagnosed in the antenatal period.
Abbreviations: TRAP: Twin Reversed Arterial Perfusion
Introduction
The acephalic acardiac twin, a particular form of transfuser-transfused syndrome. Also known as twin reversed arterial perfusion (TRAP) syndrome. It is a rare malformation, accounting for less than 1 in 35,000 births, and is seen exclusively in monochorionic twin pregnancies, affecting less than 1% of them [1]. We report a case of an acephalic acardiac fetus diagnosed on ultrasound at 18 weeks' amenorrhea (SA). Early antenatal diagnosis is essential, enabling appropriate follow-up and even treatment [2-4].
Case Report
This was a 37-year-old patient, known to be asthmatic, with 4 live children born at term by caesarean section and 3 spontaneous miscarriages. Her pregnancy was monitored by a general practitioner up to 18 weeks' gestation. A morphological ultrasound examination at 18 weeks' gestation showed a live fetus and a formation surrounded by a membrane, suggesting a monozygotic twin pregnancy with a normal live fetus and a second acephalic acardiac twin (figs 1, 2, 3 and 4). A biometric discrepancy between the 2 twins, diffuse subcutaneous oedema or morphological anomalies on the dead twin, especially as it was a monochorial biamniotic placenta. Pathological examination of the malformed fetus (fig. 4) confirmed the diagnosis of an acephalic acardiac twin. The cord of the acardiac fetus contained a single umbilical artery and was inserted into that of the donor fetus. No cardiac structure was identified, nor was there a normal vena cava system however, there was an aorta with 2 branches at its cephalic and caudal ends. Sex undetermined. Examination of the placenta revealed a monochorial biamniotic placenta.
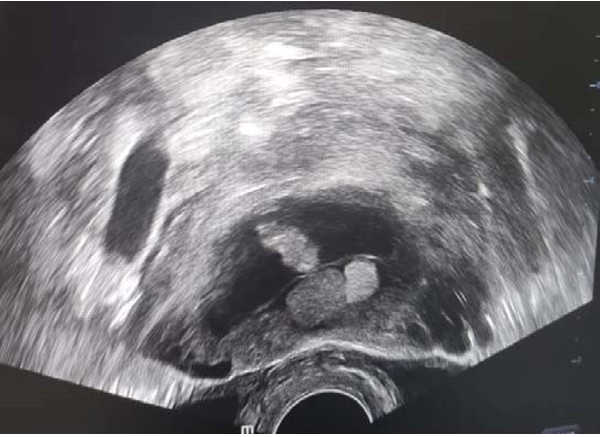
Figure 1: Ultrasound Image of an Acephalic Acardiac Twin
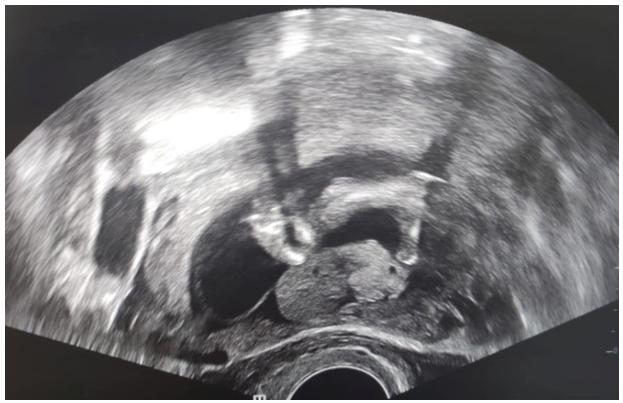
Figure 2: Ultrasound Image of an Acephalic Acardiac Twin
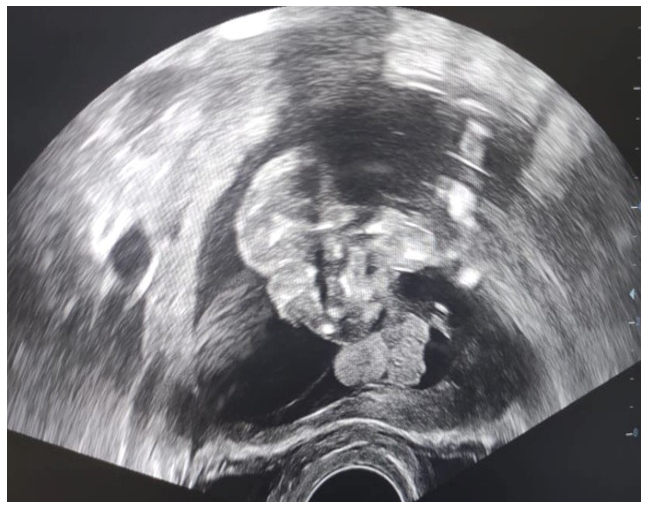
Figure 3: Ultrsasound Image of an Acephalic Acardiac Twin

Figure 4: Morphology of the Twins
Discussion
The acephalic acardiac fetus is a rare malformation, occurring exclusively in monozygotic twin pregnancies whose vascularization is ensured by a double set of vascular anastomoses allowing the normal twin to perfuse it countercurrently via an umbilical artery. The acardiac twin is no longer vascularized by the placenta, but solely by its twin [1,4]. This description corresponds to the twin reverse arterial perfusion (TRAP) syndrome of the Anglo-Saxons, a phenomenon which appears to be a particular form of the transfuser-transfused syndrome; the normal fetus being the donor, it 'delivers blood for itself and for the acardiac fetus considered' as the recipient. Venous return is via the umbilical vein of the normal twin [1-5]. In the transfusertransfused syndrome, it is the recipient who develops hydrops. Morphological anomalies of the cephalic pole, limbs and abdominal organs are linked to the interruption of organogenesis due to changes in blood flow in the acardiac twin. The reversal of blood flow alone would best explain the incomplete morphogenesis or even regression of certain existing structures, notably the fetal circulatory system. In fact, the acardiac fetus is perfused against the current with oxygen-depleted blood from an umbilical artery, often the only one (50% of cases). This deoxygenated blood, arriving from the donor at low pressure, instead of returning normally to the placenta, goes directly to the acardiac twin [1,2,4,6]. The fetopathological study by Van Allen et al [7], based on a series of 14 acardiac fetuses, showed that morphogenesis correlated with the development of the circulatory system. Subcutaneous oedema, equivalent to hydrops, is very frequent, also linked to circulatory disorders [1,4]. A karyotype abnormality is present in 50% of cases [3]. In our case, a karyotype study could not be performed. Prenatal diagnosis is possible as early as the 1st trimester of pregnancy [5,6,8]. Pulsed Doppler analysis is used to confirm the diagnosis, showing a reversal of blood flow in the umbilical vessels [7,8]. Biometric discordance between the 2 twins, diffuse subcutaneous edema or morphological abnormalities in one of the 2 twins suggest the diagnosis of an acardiac fetus. Biometric discordance is a constant and early ultrasound sign. It is visible as early as 9 SA [8]. Subcutaneous oedema is very frequently associated. Some acardiac fetuses may retain a rudimentary heart or cardiac ébauche, which explains the persistence of cardiac activity at later stages [7,8]. The differential diagnosis is made with the death of the second twin during the first weeks of pregnancy. Subsequent ultrasound scans are diagnostic in this case, showing continued growth of anencephaly, absence of facial mass, anophthalmia, cleft the acardiac fetus [4,8]. Abnormalities that should prompt a diagnosis include absence of cranium, holoprosencephaly, palate, absent or rudimentary limbs, diaphragmatic atresia, absence of thorax and heart, esophageal atresia, absence of liver and gallbladder, skin edema and a single umbilical artery. The transfusing twin should also be examined for signs of cardiac failure such as ascites, pleural effusion, hydramnios or skin edema [1,8]. Doppler examination may show flow with abnormally high resistance in the umbilical artery of the acardiac twin. This examination alone has no predictive value for the outcome of the donor fetus. The difference in resistance indices between twins appears to be best correlated with pregnancy outcome. A large difference in resistance, greater than 0.20 between the donor twin and the acardiac twin, is associated with good outcomes, whereas a difference of less than 0.05 is associated with the occurrence of complications [1,7,8]. The prognosis for the acardiac twin is fatal. Mortality of the donor twin is in the order of 50-75%, most often due to heart failure [7,9]. In our observation, as the "donor" twin did not present with heart failure, the prognosis was good. We initially opted for amniodrainage to reduce uterine contractions due to excess amniotic fluid. We believe that this wait-and-see attitude was justified given the absence of signs of heart failure in the donor fetus. Moore et al [10] have shown that the occurrence of hydramnios, preterm delivery and perinatal outcome were strongly related to the ratio of the weight of the acardiac fetus to the weight of the donor. Thus, when the acardiac twin weighs less than 25% of the donor's weight, the outcome is generally favorable. On the other hand, if the ratio is over 70%, the incidence of preterm delivery is 90%, hydramnios 40% and donor heart failure 30%, compared with 75%, 30% and 10% respectively when the ratio is lower. Indeed, the increase in perfusion required by a "large" acardiac compared with a normal twin determines the prognosis. However, estimating the weight of the acardiac twin is very difficult because of its malformations, and the measurements used were most often made retrospectively in the postnatal period. In our case, although this ratio was 80.5%, there was no heart failure in the live twin, but hydramnios. Some echographic prognostic factors have been identified. The variables studied in the healthy fetus are cardiothoracic ratio and left ventricular ejection fraction. In the acardiac fetus, they are the maximum length, length and size of cysts secondary to edema, the presence of a rudimentary heart and, in both fetuses, the pulsatility index of the umbilical artery. Thus, an increased pulsatility index of the umbilical artery of the acardiac twin compared with that of the normal twin, an increased ejection fraction in the 2nd trimester and rapid mass growth are associated with a poor prognosis. The cardiothoracic ratio and the presence of cysts have no prognostic value [7,9]. Thus, these pregnancies require regular ultrasound monitoring to look for signs of donor heart failure. Interventional treatments aim to interrupt vascular communications between the 2 twins [9,10]. Seeds et al [11] have proposed selective foeticide, but injection of a lethal substance could also affect the healthy twin. Endoscopic laser coagulation of the umbilical cord vessels of the acardiac twin under ultrasound control is an alternative, enabling complete cessation of reverse arterial perfusion and disappearance of tricuspid regurgitation in the normal twin within 2 weeks, with no subsequent complications [12,13]. Selective reduction of the acardiac twin by radiofrequency at the level of the cord insertion in the acardiac twin can be performed. A needle is inserted percutaneously to the cord origin and energy is delivered until blood flow is stopped in the acardiac twin [14]. Lewi et al [3] recently demonstrated the efficacy of clamp coagulation of the umbilical cord of the acardiac twin in 80 successful pregnancies. For Wong and Sepulveda [15]. when the acardiac twin is small, simple ultrasound monitoring is recommended, with a search for complications in the healthy twin. On the other hand, when the acardiac twin is large or growing rapidly, invasive treatment by coagulation or radiofrequency interruption of the abdominal aorta or pelvic vessels should be considered.
Conclusion
The acardiac twin is a particular and extremely rare form of transfusiontransfusion syndrome complicating a monozygotic twin pregnancy. It is diagnosed in the ante-natal period using Doppler ultrasound. Its discovery requires close monitoring of the pregnancy to detect heart failure in the the 2 twins in order to achieve selective fœticide. In practice, the obstetric course of action fluctuates between abstention donor twin, whose mortality rate remains high. The proposed treatments aim to interrupt vascular communications between and interventionism, in view of the prematurity and heart failure on which the prognosis of the healthy twin depends.
References
- Guigue V., Schweterle F., Arbez-Gindre F. Uncas de jumeau acardiaque acéphale et revue de la litérature. J Gynecol Obstet Biol Reprod. 2007;36:293–7. [Ref.]
- Fatnassi R., Mesri H., Karray T., et al. Le fœtus acardiaque acéphale: diagnose anténatal et modalités thérapeuques. Imagerie de la Femme. 2009;19:51–5. [Ref.]
- Lewi L., Gratacos E., Orbus E., et al. Pregnancy and infant outcome of 80 consecutive cord coagulations in complicated monochorionic multiple pregnancies. Am J Obstet Gynecol. 2006;194(3):782–9. [PubMed.]
- Pouehe D., Galer J-L., Rique M. Fœtus acardiaque acéphale et grossesse gémellaire: à propos d’un case. J Gynecol Obstet Biol Reprod (Paris). 1999;28(3):275–7. [PubMed.]
- Coulam CB., Wright G. First trimester diagnosis of acardiac twins. Early Pregnancy. 2000;4(4):261–70. [PubMed.]
- Kayalvizhi I., Dhall U., Magu S. Acardius acephalus monster: A case report. J Anat Soc India. 2005;54:26–8. [Ref.]
- Van Allen MI., Smith DW., Shepard TH. Twin reversed arterial perfusion (TRAP) sequence: A study of 14 twin pregnancies with acardius. Semin Perinatol. 1983;7(4):285–93. [PubMed.]
- Baron M., Verspyck E., Diguet A., et al. Sémiologie échographique du fœtus acardiaque au premier trimestre de la grossesse. J Gynecol Obstet Biol Reprod (Paris). 2000;29:684–6. [Ref.]
- Brassard M., Fouron JC., Leduc L., et al. Prognostic markers in twin pregnancies with an acardiac fetus. Obstet Gynecol. 1999;94(3): 409–14. [PubMed.]
- Moore TR., Gale S., Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163(3):907–12. [PubMed.]
- Seeds JW., Herbert WN., Richards DS. Prenatal sonographic diagnosis and management of a twin pregnancy with placenta previa and hemicardia. Am J Perinatol. 1987;4(4):313–6. [PubMed.]
- Hecher K., Reinhold U., Gbur K., et al. Interrupon of umbilical blood flow in an acardiac twin by endoscopic laser coagulaon. Geburtshilfe Frauenheilkd. 1996;56:97–100. [Ref.]
- Deprest JA., Audibert F., Van Schoubroeck D., et al. Bipolar coagulation of the umbilical cord in complicated monochorionic twin pregnancy. Am J Obstet Gynecol. 2000;182(2):340–5. [PubMed.]
- Tsao K., Feldstein VA., Albanese CT., et al. Selective reduction of acardiac twin by radiofrequency ablation. Am J Obstet Gynecol. 2002;187(3):635–40. [PubMed.]
- Wong AE., Sepulveda W. Acardiac anomaly: Current issues in prenatal assessment and treatment. Prenat Diagn. 2005;25(9): 796–806. [PubMed.]