>Corresponding Author : Benhadouga Khadija
>Article Type : Case Report
>Volume : 5 | Issue : 6
>Received Date : 30 June, 2025
>Accepted Date : 10 July, 2025
>Published Date : 14 July, 2025
>DOI : https://doi.org/10.54289/JCRMH2500126
>Citation : Benhadouga K, Fatima ZC, Elhodaigui N, Bouchane H, Benchrefi Y, Benhassou M, et al. (2025) Serous Endometrial Adenocarcinoma (SEA) and Mixed Müllerian Tumor of the Ovary (MMOT): A Report Case. J Case Rep Med Hist 5(6): doi https://doi.org/10.54289/JCRMH2500126
>Copyright : © 2025 Benhadouga K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Resident Physician, Department of Gynecology and Obstetrics, at Ibn Rochd University Hospital, Casablanca, Morocco
2Professor in the Department of Gynecology and Obstetrics at the Ibno Rochd University Hospital in Casablanca, Morocco
*Corresponding author: Benhadouga Khadija, Resident Physician, Department of Gynecology and Obstetrics, at Ibno Rochd University Hospital, Casablanca, Morocco
Abstract
Serous endometrial adenocarcinoma (SEA) and mixed mullerian tumor of the ovary (MMOT) are two rare, highly aggressive tumor entities arising from the mullerian epithelium. Despite their distinct localization, they share many morphological, immunohistochemical and molecular features, including a high frequency of TP53 mutations, loss of hormonal expression and a high-grade serous signature. Their prognosis is unfavorable, largely due to late diagnosis, early dissemination and limited response to standard treatments. Cytoreductive surgery and platinum-based chemotherapy are the mainstays of treatment, although their efficacy remains limited, particularly in TMMO. However, recent advances in molecular biology are paving the way for targeted therapeutic approaches, including immunotherapy. A better understanding of the biology of these tumors will help optimize their management and improve patient survival.
Keywords: Serous Adenocarcinoma; Endometrium; Mixed Mullerian Tumor; Ovarian Carcinosarcoma; TP53; Immunotherapy; Gynecological Cancer
Abbreviations: SEA: Serous Endometrial Adenocarcinoma, MMOT: Mixed Mullerian Tumor of the Ovary, EMT: Epithelial-Mesenchymal Transition, MSI: Microsatellite Instability
Introduction
Serous endometrial adenocarcinoma (SEA) and mixed Müllerian tumor of the ovary (MMOT), also known as ovarian carcinosarcoma, are two rare but highly aggressive gynecological cancers. ESA accounts for around 10% of endometrial cancers, but is responsible for almost 40% of deaths due to this pathology [1]. This high-grade carcinoma is characterized by papillary architecture, strong nuclear atypia and a marked tendency to ectopic dissemination, even at an early stage [2].
Mixed Mullerian tumors of the ovary are rare biphasic neoplasia, accounting for less than 1% of all ovarian tumors [3]. They combine a carcinomatous epithelial contingent (often high-grade serous) with a sarcomatous mesenchymal contingent, giving them a particularly aggressive clinical behaviour [4].
Despite their distinct locations, ASE and TMMO share morphological and molecular similarities, including frequent mutation of the TP53 gene and chromosomal instability [5,6]. Their comparison raises important questions about the pathogenesis, diagnostic criteria and therapeutic implications of these tumors arising from the müllerian genital tract.
Case Report
This 48-year-old patient, hypertensive and on treatment for 3 years, presented with abnormal uterine bleeding dating more than 2 years. On clinical examination: an abdomino-pelvic mass reaching midway to the umbilicus, anterior cervix retracted by the mass, bleeding of endo-uterine origin with perception of the lower pole of the mass on vaginal touch.
Pelvic ultrasound: (Figure 1-2)
- Uterus measuring 74x50x69 mm
- Right ovary with cystic image; heterogeneous, septate with highly suspicious, vascularized septa measuring 99x88 mm
- Left ovary: an anechogenic thin-walled cyst measuring 49 mm
Abdominal-pelvic MRI: (Figure 3-4-5)
- Uterus increased in volume by 136 mm
- Highly suspicious endometrial tumour process infiltrating more than half the myometrium, respecting the serosa measuring 96 mm
- Left retro- and latero-uterine mass with cystic portions and a tissue portion, extending posteriorly and inferiorly through a fluid formation and narrowing the rectosigmoid.
- 45mm right liquid mass
- ADP right external iliac, left internal iliac, left latero-aortic of 2 cm
- Medium-volume effusion
CA 125: 95.6 U/mL
The patient underwent biopsy of the endo-cavitary endometrial mass + biopsy of a nodule in the lower 1/3 of the vagina + biopsy of the ovarian mass + peritoneal and epiploic biopsy + cytology of the ascites fluid + cytology of the ovarian mass aspiration fluid. Anatomopathological study: infiltration of the endometrium and lower 1/3 of the vagina by a serous, infiltrating adenocarcinoma poorly differentiated from the endometrium.
Morphological appearance suggestive of carcinosarcoma (mixed malignant molar tumor) of the ovary. Presence of vascular emboli.
biopsy showing hemorrhagic and congestive infammatory changes, with no signs of specificity or malignancy.
Omentectomy infiltrated by a poorly differentiated adenocarcinoma.
Cytology of ascites fluid and ovarian mass contents containing tumour cells.
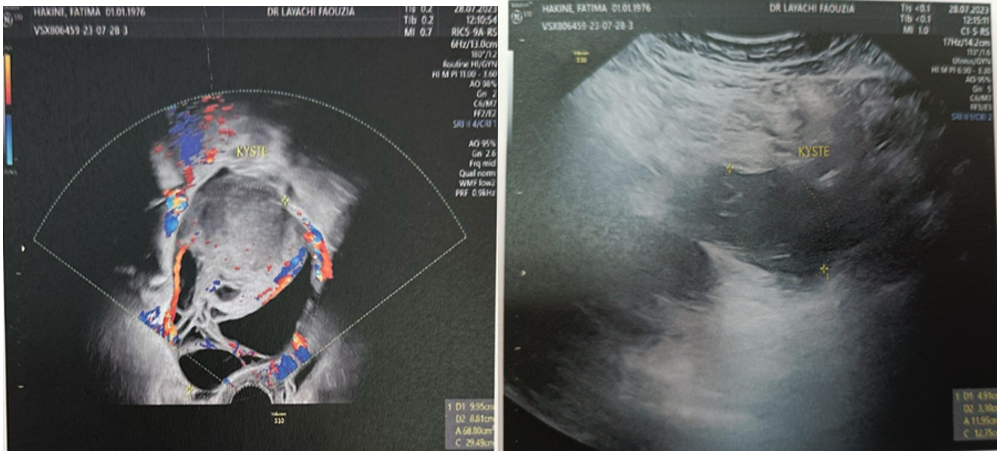
Figure 1-2: Ultrasound image of the ovarian mass.
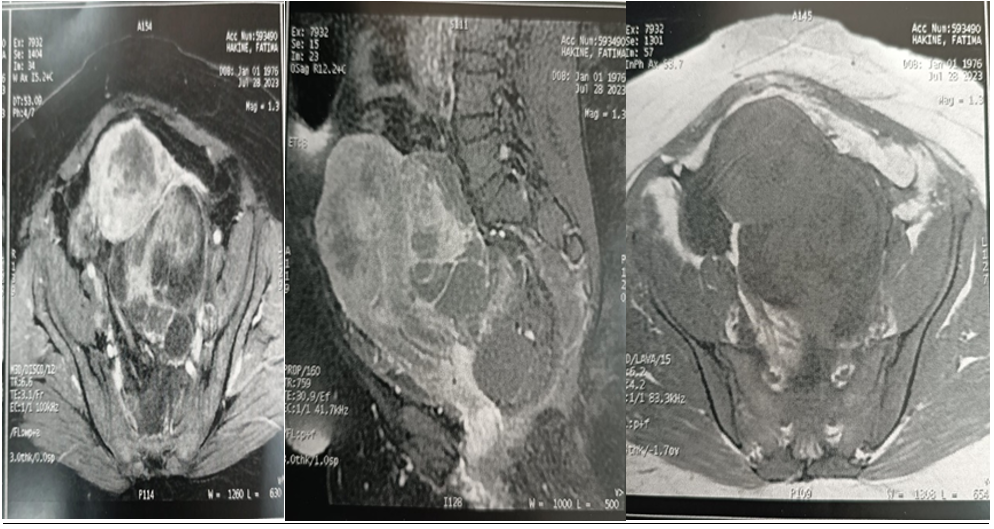
Figure 3-4-5: Ultrasound image of the ovarian mass.
Discussion
Serous endometrial tumors and mixed mullerian ovarian tumors share a common biology marked by genomic instability, TP53 mutations in over 80% of cases and a transcriptomic signature close to high-grade serous carcinomas of the ovary [5,7]. In ESA, loss of hormone receptor expression, diffuse p16 expression and high Ki-67 index reflect rapid, hormone-axis-independent tumor proliferation [2].
TMMO, although rarer, add morphological complexity through the presence of a homologous (composed of endometrial or myogenic tissue) or heterologous (containing cartilage, bone or striated muscle) sarcomatous contingent [8]. This biphasicity probably results from a process of epithelial-mesenchymal transition (EMT), contributing to greater aggressiveness [9].
The prognosis is poor for both entities. The 5-year survival rate for ESA is less than 50%, even with optimal treatment [1]. For TMMO, median survival varies from 12 to 24 months depending on the series, irrespective of the stage at diagnosis [3,10].
Therapeutic management is mainly based on complete excision surgery. In ESA, total hysterectomy with bilateral adnexectomy, pelvic and para-aortic curage is recommended [11]. Adjuvant chemotherapy with carboplatin and paclitaxel improves recurrence-free survival [12]. For BMMT, optimal cytoreduction followed by chemotherapy is essential, although the sarcomatous component responds poorly to standard treatments [4].
Recent advances suggest a growing interest in targeted therapy. Immunotherapy, notably PD-1/PD-L1 inhibitors, has shown promising results in early trials in patients with microsatellite instability (MSI) or high mutational load mullerian tumors [13].
In short, although ESA and TMMO are distinct entities, their biological similarities underscore the need for further research to tailor treatments to their specific molecular profiles.
Conclusion
Serous adenocarcinoma of the endometrium and mixed Müllerian tumours of the ovary represent two rare but particularly formidable forms of gynaecological cancer. Their aggressive nature, poor response to conventional treatments and tendency to spread rapidly justify special attention, both diagnostically and therapeutically. The convergence of their molecular profiles, in particular TP53 alterations, suggests a biological kinship that could be exploited to develop common therapeutic strategies. Targeted therapies and immunotherapy, although still in the evaluation phase, offer promising prospects. It is essential to promote translational research and clinical trials dedicated to these rare entities in order to improve their long-term prognosis.
Reference
- Forney JP., Buschbaum HJ. Classifying staging and treating uterine sarcomas. Contemporary Ob Gyn. 1981;18:47–50. [Ref.]
- Seddon BM., Davda R. Uterine sarcomas — recent progress and future challenges. Eur. J. Radiol. 2011;78:30–40. [PubMed.]
- Philip PC., Annie Cheung NY. Pathology of uterine leiomyosarcomas and smooth muscle tumours of uncertain malignant potential. Best Pract. Res. Clin. Gastroenterol. 2011;25, 691–704. [PubMed.]
- Paudel P., Dhungana B., Shrestha E., et al. Leiomyosarcoma of the Uterus: A Rare Diagnosis. Cureus 2021;13(8):e17418. [PubMed.]
- George S., Serrano C., Hensley ML & Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. Journal of Clinical Oncology. 2018;36(2):144–150. [PubMed.]
- Cao S., Liu Y., Bai X., Wang L. A case report of uterine leiomyosarcoma. OncoTargets and Therapy. 2019;12:8583–8586. [PubMed.]
- Forney JP, Buschbaum HJ. Classifying staging and treating uterine sarcomas. Contemporary Ob Gyn. 1981;18:47–50. [Ref.]
- Juhasz-Böss I., Gabriel L., Bohle RM., Horn LC., Solomayer EF., Breitbach GP. Uterine leiomyosarcoma. Oncol Res Treat. 2018;41:680–686. [PubMed.]
- Kobayashi H., Uekuri C., Akasaka J., Ito F., Shigemitsu A., et al. The biology of uterine sarcomas: a review and update. Mol Clin Oncol. 2013;1:599–609. [PubMed.]
- Roberts ME., Aynardi JT., Chu CS. Uterine leiomyosarcoma: a review of the literature and update on management options. Gynecol Oncol. 2018;151:562–572. [PubMed.]
- Santos P., Cunha TM. Uterine sarcomas: clinical presentation and MRI features. Diagn Interv Radiol. 2015;21:4–9. [PubMed.]
- Hadoux J., Morice P., Lhommé C., et al. Uterine leiomyosarcoma: epidemiology, pathology, biology, diagnosis, prognosis and treatment. Bull Cancer. 2013;100:903–915. [PubMed.]
- Bacanakgil BH., Deveci M., Karabuk E., Soyman Z. Uterine smooth muscle tumor of uncertain malignant potential: clinicopathologic-sonographic characteristics, follow-up and recurrence. World J Oncol. 2017;8:76–80. [PubMed.]