>Corresponding Author : Zineb El Jaouhari
>Article Type : Case Report
>Volume : 5 | Issue : 4
>Received Date : 21 April, 2025
>Accepted Date : 03 May, 2025
>Published Date : 12 May, 2025
>DOI : https://doi.org/10.54289/JCRMH2500117
>Citation : El Jaouhari Z, Mounssif A, Belmalyani R, Bouziane M, Haboub M, et al. (2025) Endocardial Dissection After MI: A Rare Complication with Diagnostic Complexity. J Case Rep Med Hist 5(4): doi https://doi.org/10.54289/JCRMH2500117
>Copyright : © 2025 El Jaouhari Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
Department of Cardiology, Chu Ibn Rochd Casablanca Morocco
*Corresponding author: Zineb El Jaouhari, Department of Cardiology, Chu Ibn Rochd Casablanca Morocco
Abstract
Background: Endocardial dissection is an extremely rare and underrecognized complication of myocardial infarction (MI), involving the separation of the endocardial layer from the underlying myocardium. This condition is often misdiagnosed due to its overlap with other intracardiac abnormalities such as pseudoaneurysms, mural thrombi, or intramyocardial dissecting hematoma (IDH).
Case Report: We present the case of a 67-year-old male smoker admitted with acute anterior myocardial infarction, characterized by chest pain and ST-segment elevation on electrocardiogram. He underwent successful thrombolysis followed by rescue angioplasty with stent placement in the proximal left anterior descending artery. Despite initial stabilization, the patient experienced progressive dyspnea and peripheral edema one month later. Laboratory tests revealed elevated biomarkers, and transthoracic echocardiography (TTE) identified a hypoechoic space measuring 60 × 55 mm in the left ventricle, consistent with an endocardial dissection and dissecting hematoma. Despite surgical recommendations, the patient was managed conservatively due to family preferences and was discharged after symptomatic improvement.
Conclusion: This case highlights the diagnostic and therapeutic challenges associated with endocardial dissection, a rare post-MI complication. The use of TTE and other imaging modalities is crucial for accurate diagnosis, distinguishing it from other conditions with similar presentations. The lack of established treatment guidelines underscores the need for further case studies to better understand its pathophysiology, clinical course, and management options. Enhanced awareness and reporting are essential for improving outcomes in patients with this rare condition.
Keywords: MI, Endocardic, Dissection
Abbreviations: MI: Myocardial Infarction, IDH: Intramyocardial Dissecting Hematoma, TTE: Transthoracic Echocardiography, BNP: B-Type Natriuretic Peptide, CRP: C-Reactive Protein, LVEF: Left Ventricular Ejection Fraction, ECG: Electrocardiogram
Introduction
Endocardial dissection is an uncommon mechanicalcomplication that can occur after myocardial infarction (MI). This condition involves the detachment of the endocardiallayer from the underlying myocardial tissue and is often categorized as a form of incomplete or contained rupture. Due to its rarity and limited documentation in the medical literature, endocardial dissection is frequently misdiagnosed or overlooked. The clinical spectrum varies significantly, ranging from cases with no symptoms to severe and potentially fatal outcomes. This report discusses a case of endocardial dissection identified through echocardiographic imaging, emphasizing its diagnostic complexities and potential treatment considerations.
Case Presentation
A 67-year-old man patient with a prior history of smoking, was admitted for intermittent chest tightness and pain of three hours duration. Physical examination showed a heart rate was 85 bpm, the respiration was 20 bpm, and the blood pressure was 150/118mmHg. The emergency 18-lead emergency electrocardiogram (ECG) showed ST-segment elevation in the extensive anterior wall leads (V1-V6, I, aVL) (Fig1). Laboratory tests revealed a significantly elevated cardiac troponin level at 20,000 ng/L. Renal function was within normal limits, and C-reactive protein (CRP) was markedly elevated, reflecting an inflammatory response. The remainder of the laboratory findings, including electrolytes, liver enzymes, and hematological parameters, were unremarkable.
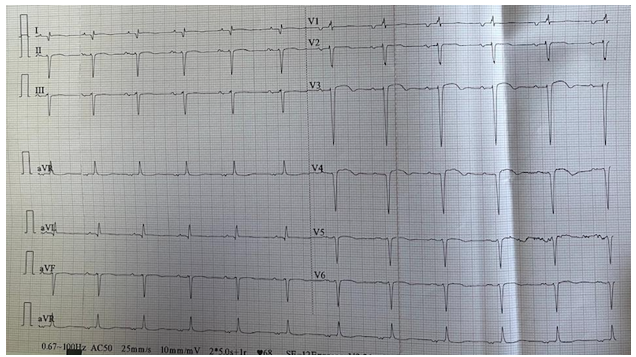
Figure 1: ECG showed ST-segment elevation.
He successfully underwent weight-adjusted thrombolysis. The rescue angioplasty performed at H12 revealed significant stenosis of approximately 80–90% in the proximal and proximal-mid-segment of the opening of the anterior descending artery along with its diagonal branch orifice. Two stents were implanted after balloon pre-dilatation of the stenotic lesions in the proximal and proximal-mid-segment of the opening of the anterior descending branch via the right radial artery pathway, and high-pressure balloon dilatation was performed with no residual stenosis, and TIMI 3 flow.
TTE showed the hypokinesis of the anterior and inferior septum, anterior wall with a calculated (LVEF) at 37% (Figure 2). Dual antiplatelet therapy (DAPT) consisting of aspirin plus clopidogrel was administered postoperatively in addition to (HF) treatment. He was discharged after stabilisation.
One month after, the patient experienced worsening dyspnea accompanied by lower limb edema. Clinical examination found a blood pressure of 105/83 mmHg and a heart rate of 82 bpm. He exhibited crepitant rales extending from the lung bases to the midfields, accompanied by edema reaching up to the legs.
Laboratory tests revealed elevated levels of BNP (1798pg/ml), Troponine (226ng/ml versus 194 ng/ml) and CRP (85.6mg/ml).
The ECG showed a sinus rythm, with persistent anterior wall ST elevation segments and T waves inversion.
TTE showed hypokinetic segments including the anterior septum, anterior wall, lateral wall, and apical akinesia. The fourchamber view showed an low echo area space measuring 60 × 55 mm sandwiched between the endocardium with no evident color flow signal on color Doppler imaging (Fig3).
These findings suggested a left ventricular endocardic dissecting hematoma. Despite the cardiothoracic surgeon’s recommendation for surgical intervention, the patient’s family refused. Ultimately, the patient was discharged again after symptom relief (Fig 4).
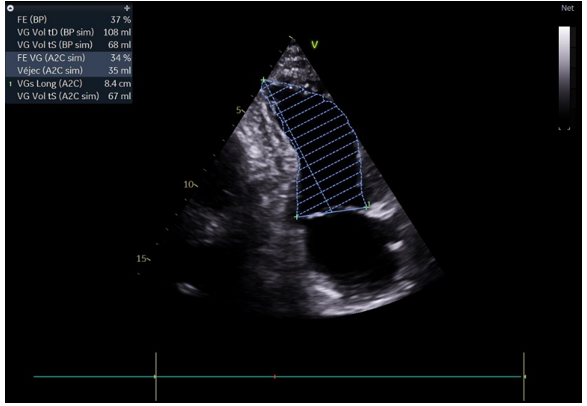
Figure 2: TTE showed the LVEF at 37%
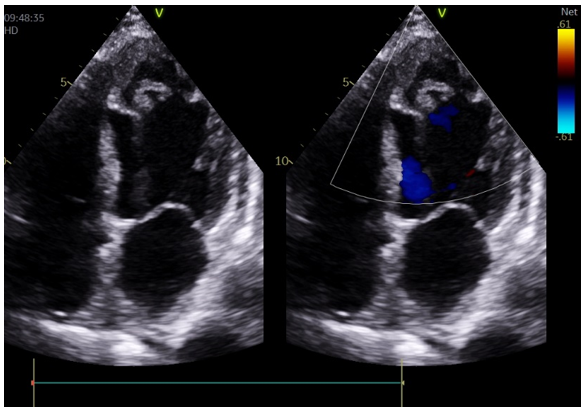
Figure 3: The low echo area space sandwiched between the endocardium with no evident color flow signal on color Doppler imaging
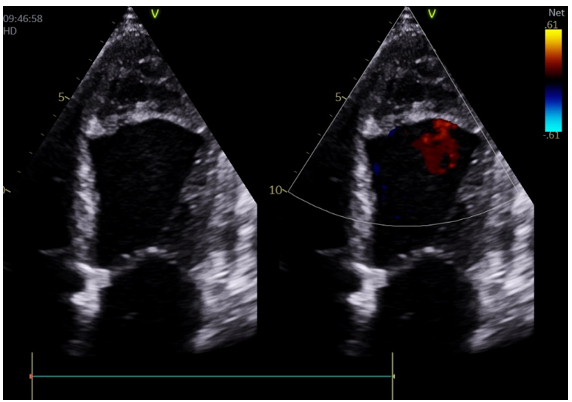
Figure 4: left ventricular endocardic dissecting hematoma
Discussion
Typically, following a myocardial infarction, the affected area of the myocardium undergoes full-thickness necrosis, leading to thinning and expansion of the ventricular wall. Over time, the necrotic tissue is replaced by fibrous scar tissue, resulting in a loss of normal contractile function [1].
Under the pressure generated by ventricular contraction, the infarcted ventricular wall may protrude outward, forming a ventricular aneurysm. In rare instances, like our case, a subendocardial myocardial infarction (non-transmural) can occur, where necrosis and rupture are confined to the inner layers of the myocardium near the endocardium [2,3]. In such cases, high-pressure blood flow within the left ventricle can exert force on the weakened myocardium, causing it to tear. This allows blood to infiltrate between the myocardial layers, forming a dissection and hematoma, while the epicardium remains intact.
Endocardial dissection, a rare and poorly understood complication of myocardial infarction (MI), presents significant challenges in diagnosis and management, given its limited documentation in the medical literature. Unlike intramyocardial dissecting hematoma (IDH), [4]. which involves a hemorrhagic separation of myocardial fibers and has been better studied, endocardial dissection specifically refers to the delamination of the endocardium from the myocardium. The distinction between these two entities is subtle yet critical, as their clinical behavior and management strategies differ.
However, comparable data for endocardial dissection are lacking, leaving treatment approaches largely empirical and dependent on individual case factors. The diagnostic overlap between endocardial dissection and other intracardiac conditions, such as pseudoaneurysms, [5] mural thrombi, or ventricular rupture, further complicates early recognition. Studies on IDH have highlighted the frequent misdiagnosis of such complications, often requiring advanced imaging modalities like cardiac MRI or contrast-enhanced CT to accurately characterize structural abnormalities [6].
In our case, TTE was instrumental in identifying the dissection, underscoring its value in distinguishing rare conditions from more common post-MI complications [7]. Additionally, while the literature on IDH has noted spontaneous resolution in cases involving heightened fibrinolytic activity, particularly in patients with malignancy or other systemic factors, similar mechanisms have not been confirmed for endocardial dissections, [8] leaving their pathophysiological underpinnings speculative.
This highlights the urgent need for further case reports and studies to elucidate the clinical trajectory and optimal management strategies for this rare and potentially life-threatening condition, which remains a diagnostic and therapeutic frontier in post-MI care.
Conclusion
Endocardial dissection following myocardial infarction represents an exceptionally rare and underrecognized complication with significant diagnostic and therapeutic challenges. This unique condition is characterized by a lack of specific clinical symptoms and diverse presentations, requiring advanced imaging modalities for accurate diagnosis. Although cases of intramyocardial dissecting hematoma have provided insights into potential mechanisms, including high-pressure blood flow and myocardial tearing, similar data for endocardial dissections remain scarce.
This case underscores the importance of vigilant imaging assessment, multidisciplinary management, and the need for further research to better understand the pathophysiology, natural history, and optimal treatment strategies for this rare complication. Enhanced awareness and reporting of such cases will be crucial in improving outcomes and guiding future management protocols.
Declarations
Consent to participate: All participants provided written informed consent during the study.
Ethics approval: The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, 1975. The Ethical Committee of Faculty of Medicine, Hassan II University approved the study.
Consent for Publication: Not applicable
Clinical Trial Number: Not applicable
Funding: Not applicable
References
- Mulvagh SL., Rakowski H., Vannan MA., Abdelmoneim SS., Becher H., Bierig SM., et al. American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J Am Soc Echocardiogr. Nov 2008; 21(11):1179‒201. [PubMed.]
- Yue R., Peng Y., Xu W. A Case Report of Intramyocardial Dissecting Hematoma Similar to Left Ventricular Mural Thrombus. 2024;08(04):83‒9. [Ref.]
- Dias V., Cabral S., Gomes C., Antunes N., Sousa C., Vieira M., et al. Intramyocardial dissecting haematoma: a rare complication of acute myocardial infarction. Eur J Echocardiogr. juin 2009;10(4):585‒7. [PubMed.]
- Leitman M., Tyomkin V., Sternik L., Copel L., Goitein O., Vered Z. Intramyocardial dissecting hematoma: Two case reports and a meta-analysis of the literature. Echocardiography. Févr 2018;35(2):260‒6. [PubMed.]
- Nguyen EK., Suksaranjit P., Bashir MA., Firchau DJ., Gebska MA. Decoding Postinfarction Left Ventricular Pseudoaneurysm. JACC Case Rep. 2022;9:101533. [PubMed.]
- Roslan A., Jauhari Aktifanus AT., Hakim N., Megat Samsudin WN., Khairuddin A. Intramyocardial Dissecting Hematoma in Patients with Ischemic Cardiomyopathy: Role of Multimodality Imaging in Three Patients Treated Conservatively. CASE (Phila). 2017;1(4):159‒62. [PubMed.]
- Roslan A., Jauhari Aktifanus AT., Hakim N., Megat Samsudin WN., Khairuddin A. Intramyocardial Dissecting Hematoma in Patients with Ischemic Cardiomyopathy: Role of Multimodality Imaging in Three Patients Treated Conservatively. CASE (Phila). août 2017;1(4):159‒62. [PubMed.]
- Spinelli L., Stabile E., Giugliano G., Morisco C., Giudice CA., Imbriaco M., et al. Intramyocardial dissecting hematoma in anterior wall ST elevation myocardial infarction: impact on left ventricular remodeling and prognosis. Int J Cardiovasc Imaging. Févr 2018;34(2):201‒10. [PubMed.]