>Corresponding Author : Errih Leila
>Article Type : Case Report
>Volume : 5 | Issue : 3
>Received Date : 08 April, 2025
>Accepted Date : 18 April, 2025
>Published Date : 05 May, 2025
>DOI : https://doi.org/10.54289/JCRMH2500112
>Citation : Leila E, Fadwa B, Ezzahra BF, Amine L, Mohamed J, et al. (2025) Gestational Choriocarcinoma: Report Case. J Case Rep Med Hist 5(3): doi https://doi.org/10.54289/JCRMH2500112
>Copyright : © 2025 Leila E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Resident Physician, Department of Gynecology and Obstetrics, at Ibno Rochd University Hospital, Casablanca, Morocco
2Professor in the Department of Gynecology and Obstetrics at the Ibno Rochd University Hospital in Casablanca, Morocco
*Corresponding author: Errih Leila, Resident Physician, Department of Gynecology and Obstetrics, Ibno Rochd University Hospital, Casablanca, Morocco
Introduction
Gestational trophoblastic disease is a number of different clinical and pathological entities [1]. Treatment of complete and partial hydatidiform mole involves evacuation of the uterus by aspiration, and requires monitoring of hCG levels. In the event of a diagnosis of gestational trophoblastic tumour (GTT), based on non-normalisation of hCG or a histological appearance of choriocarcinoma, medical treatment will be required [2].
Observation
Mrs G S aged, 30 years mother of 1 children, without particular pathological antecedents, followed for mole hydatiforme for 2 years; she was aspirated twice with histological examination was in favour of a stopped pregnancy. Given the persistence of metrorrhagia, the patient was referred to us for management. Gynaecological examination revealed minimal bleeding from the endocervix, and the uterus was enlarged halfway to the umbilicus, with infiltrated parameters on rectal examination.
Pelvic ultrasound revealed a heterogeneous intrauterine mass measuring 5x4.5 cm. The BHCG was greater than 300,000 IU/L.
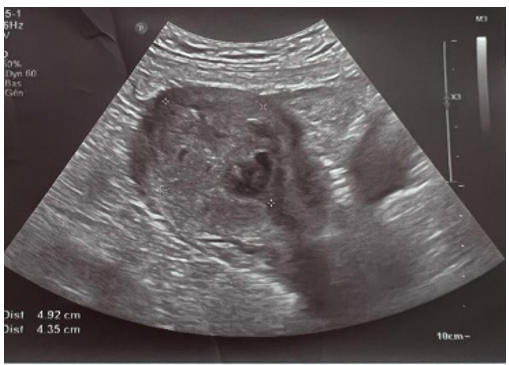
Figure 1. Ultrasound appearance of a hydatidiform mole
The patient underwent a third aspiration; histological examination revealed a remodelled choriocarcinoma with extensive necrosis. An extension work-up was carried out, showing no associated secondary lesions.
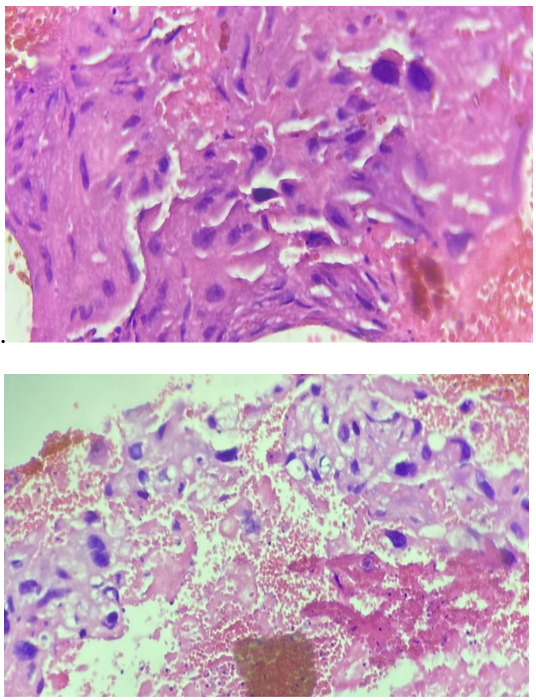
Figure 2. Histological appearance compatible with choriocarcinoma
Discussion
Choriocarcinoma is a malignant tumour of the placenta, developing at the expense of the epithelium of the ovarian chorion and invading the maternal body. The discovery of antigenic markers in choriocarcinoma that are not present in hydatidiform mole may explain why choriocarcinoma is considered to be a malignant tumour of the placenta. influences the chorial epithelium to proliferate in the manner of a malignant tumour [3]. The mechanism by which an allogeneic graft (the only one of its kind) is not rejected involves consultation with an associated immunodepression. This could explain why no intra-uterine trophoblastic residue is often found in patients with metastatic disease [4].
Traditionally, gestational trophoblastomas have been divided into hydatidiform mole, invasive mole and choriocarcinoma. Such a classification does not currently appear to be very useful, as the histological diagnosis of invasive mole and choriocarcinoma is rarely made, and chemotherapy is often started without taking account of the results of the pathological examination [5].
As a general rule, choriocarcinoma follows hydatidiform mole. Choricarcinoma is most often recognised during biological monitoring of the aftermath of a mole in the absence of any clinical signs. Clinical signs, when present, generally characterise an advanced stage [6].
Haemorrhage is the first and most important symptom.
Haemorrhage is the first and most important symptom, either simply prolonging the losses following the molar abortion, or reappearing after a latent period of a few days, weeks or months [7]. Painless, irregular and, above all, repeated, it takes on its full importance because of the history of moles [8].
General signs are delayed: anaemia, weight loss, shortness of breath.
To the touch, the uterus is slightly enlarged, too soft in consistency, with a sometime’s open neck. Ovarian cysts, similar to those that accompany mole are seen inconstantly next to the uterus. Their date of appearance varies. Contemporaneous with mole or following abortion, they may decrease or increase rapidly, without any prognostic argument being drawn from their variations [9].
Apart from haemorrhage, uterine or pelvic infection, torsion of a luteinic cyst, local destruction of the myometrium up to and including uterine perforation and intraperitoneal haemorrhage, all of which are very rare, metastases are the usual complications of choriocarcinoma [10]. Pulmonary metastases are the most common.
Other sites are rarer, but all organs and tissues may be involved: brain, liver, kidney, cervix, intestine, bone marrow, femur, gums, subcutaneous tissue, etc. These sites may require additional investigations: ultrasound, hysteroscopy, CT scan, pelvic arteriography. If left untreated, metastases progress inexorably towards extension and death. They are even particularly terebrating and highly malignant [11].
Medical chemotherapy is the mainstay of treatment for choriocarcinoma because of the particularly good results obtained in these types of malignant tumour. Treatment can only be curative. Treatment is curative [12].
Two drugs are used: methotrexate and actinomycin D
Hysterectomy from the outset is becoming increasingly rare.
Hysterectomy is indicated in cases of life-threatening uterine haemorrhage and uterine tumours that have not been eradicated by chemotherapy. Finally, some consider that hysterectomy is still indicated as an initial treatment in the pre-menopausal period, provided that there are no metastases. Chemotherapy should be used if the level of prolanuria is not reversed after excision [13].
Excision of a pulmonary metastasis should be considered when a single pulmonary image persists after long chemotherapy and hormonal excretion does not return to normal. This may result in cure [14].
Conclusion
Gestational choriocarcinoma is a rare malignancy with high metastatic potential. We report the case of a 31-year-old patient with gestational choriocarcinoma revealed by renal and pulmonary metastases, one year after a normal pregnancy. The diagnosis was made by pathological analysis of the nephrectomy specimen. The outcome was favourable after appropriate treatment with multidrug therapy. This case highlights the polymorphic symptomatology of choriocarcinoma and emphasises the importance of the βHCG assay for diagnosis [14].
Bibliographie
- Achour M., El Bakkali A., Bekkay M. Choriocarcinome sur grossesse extra-utérine. Maroc Med. 1983;5:3. [Ref.]
- Acosta-Sison H. Changing attitudes in the management of hydatiform mole Amer. J. Obstet. Gynec. 1964;88:634–636. [PubMed.]
- Amiel JL. Traitement et guérison du choriocarcinome placentaire. Progrès en Obstétrique (JV. VARANGOT) Flammation Edit. Paris. 487–1970. [Ref.]
- Amiel JL. Traitement des tumeurs placentaires in le Praticien face aux problèmes du cancer (G. MATHE) Expansion scientifique, édit;Paris P. 133–1974. [Ref.]
- Amiel JL., Roger M. Pour un meilleur contrôle des tumeurs placentaires. Now–Press–Med. 5:865–1976. [Ref.]
- Bagshawe Kd. Lawler Sd. Unmasking moles. Br. J. Obstet. Gynecol. 1982;89:255–257. [PubMed.]
- Bagshawe KD., Wilson H., Dublon P., Smith A., Baldwin M., Kardana A. Follow up after hydatiform môle, studies using radioimmuno assay for primary human chorionicGonadotrophin (H.C.G) J. Obstet–Gynec–Brit–Cwlth. 810:461–1973. [PubMed.]
- Bagshawe Kd., Webb J. Hydatiform môle in England and Wales 1973–1983. Lancet. Dent J. 1986;673–677. [PubMed.]
- Beischer NA. Hydatiform môle with coexistent fœtus. Aust. N Z J Obstet–Gynaec. 6:127–1966. [PubMed.]
- Beisher NA., Bettinger HF., Fortune DW., et al. Hydatiform mole and its complications in the state of Victoria. J Obstet–Gynaec–Brit–Cwlth. 77;263–1970. [PubMed.]
- Bomsel-Helmreich O. Expérimental heteroploïdy in Mammals, in Gropp A. and Bernirschke K. Editors: Current topies in Pathology Sring er Verlag. 1976;62:155. [Ref.]
- Brewer JI., Torok EE., Welster A., et al. Hydatiform môle: a follow up regimen for identification of invasive mole and choriocarcinoma and for selection of patients for treatment. Am J Obstet–Gynec 101:557–1968. [PubMed.]
- Brewer JI., Et Gerbie AB. Early dévelopment of choriocarcinoma in choriocarcinoma (Ed J L P. Holland et M.M. Ureschyschyn) Springer– Verlag, Berlin. 1966;94(5):692-710. [PubMed.]
- Chesley LC., et al. Hydatiform mole with special réference to recurrence and associated eclampsia Amer J Obstet Gyn. 1946;52:311–320. [PubMed.]