>Corresponding Author : Ziad Imane
>Article Type : Case Report
>Volume : 4 | Issue : 11
>Received Date : 11 June, 2024
>Accepted Date : 25 June, 2024
>Published Date : 03 Oct, 2024
>DOI : https://doi.org/10.54289/JCRMH2400156
>Citation : Tossi S, Ziad I, Elkaroini D, Boufettal H, Mahdaoui S, et al. (2024) Pure Colloid Carcinoma of the Breast: One Case Report. J Case Rep Med Hist 4(11): doi https://doi.org/10.54289/JCRMH2400156
>Copyright : © 2024 Tossi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Resident Physician, Department of Gynecology and Obstetrics, at Ibn Rochd University Hospital, Casablanca, Morocco
2Professor in the Department of Gynecology and Obstetrics at the Ibn Rochd University Hospital in Casablanca, Morocco
*Corresponding author: Ziad Imane, Resident Physician, Department of Gynecology and Obstetrics, at Ibn Rochd University Hospital, Casablanca, Morocco
Abstract
Colloid carcinoma of the breast, also known as mucinous or gelatinous carcinoma, is a rare histological form of cancer accounting for 1 to 6% of all breast cancers. It is characterized by the production of extracellular mucus. Histologically, 2 types of colloid carcinoma are distinguished: pure colloid carcinoma, in which there is no infiltrating ductal carcinoma component, and mixed colloid carcinoma, which associates foci of infiltrating ductal carcinoma with the colloid component.
Keywords: Pure Colloid Carcinoma; Surgery-Case Report; Breast Cancer
Abbreviations: MC: Mucinous carcinoma, WHO: World Health Organization
Introduction
Mucinous carcinoma (MC) of the breast or colloid carcinoma is a rare histological form, accounting for 1 to 7% of all infiltrating carcinomas of the breast [2]. According to the World Health Organization (WHO), it is defined by the presence of malignant mucus-secreting tumor cells floating in mucus. Most authors agree in distinguishing two types of colloid carcinoma: pure colloid carcinoma, in which there is no or a minority ≤ 10% infiltrating ductal carcinoma component, and mixed colloid carcinoma, which associates foci of infiltrating ductal carcinoma alongside the colloid component [3]. This subdivision is justified by the prognosis: the pure form is characterized by the presence of tumor tissue completely surrounded by abundant extracellular mucus, forming a mechanical barrier that attenuates cell invasion, making this form less aggressive and giving it a more favorable prognosis than the mixed form, which is similar to that of invasive ductal carcinoma [4].
Case presentation
51-year-old patient with a pathological history of luminal infiltrating B breast carcinoma of the left breast, HER-negative, treated with surgery radiotherapy chemotherapy hormonotherapy, admitted for management of a left breast nodule that had been evolving for 3 months. Clinical examination revealed a 2x2 cm nodule at the junction of the upper right quadrants, clinically classified T1N0MO. Mammography revealed a well-limited, rounded opacity in the right upper quadrant, with irregular, blurred contours and fine, heterogeneous spicules, with no microcalcifications. This opacity is classified as ACR 4 (Figure1). Complementary ultrasonography revealed a poorly defined, spiculated, hypoechoic, heterogeneous lesion in the right upper external quadrant, measuring20 mm in long axis, with no significant adenopathy (Figure 2).
Microbiopsy of the nodule showed pure mucinous colloid carcinoma of SBR grade I without vascular emboli. Extension workup was unremarkable. The nodule was treated conservatively, with histological results indicating pure mucinous carcinoma, SBR grade I, measuring 1.4 cm, and no carcinoma in situ (Figure 3). Lymph node dissection yielded 17 lymph nodes, 08 of which were metastatic without vascular emboli or capsular rupture. The tumor was classified as p T1N2Mo. Estrogen receptor labelling was 80%, progesterone receptor labelling was 100%, HER2 was negative, and Ki 67 was 28%. Chemotherapy has been started and radiotherapy and hormone therapy are planned.
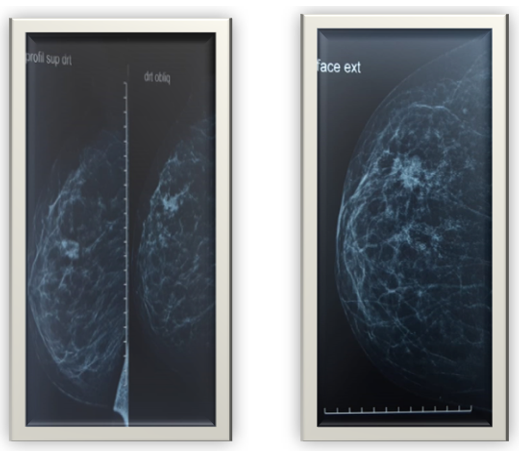
Figure 1. Mammographic appearance of the lesion.
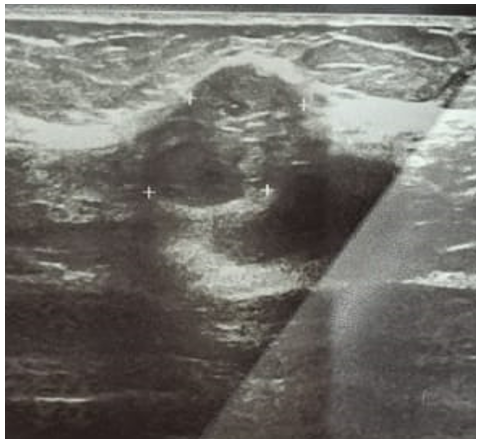
Figure 2. Ultrasound appearance of the lesion

Figure 3. Histological aspect of a colloid carcinoma of the breast.
Discussion
Colloid carcinoma is a rare histological entity of breast carcinoma, first described in 1982 by Geschickter [5]. It is a histological type that accounts for 7% of all malignant tumors of the breast after age 75 and 1% before age 35 [6].
In the literature, no significant difference has been found between colloid carcinoma, tubular carcinoma and breast CCI [7]. Pure colloid carcinomas often present as well-limited, mobile, even lobulated masses [8], which may thus be mistaken for benign formations; a sensation of suffler, crease or fluctuation is reported when colloid carcinomas of the breast are palpated [9]. The average tumor diameter is 1.5 cm, with extremes ranging from 0.3 to 19 cm [14]. The majority of tumors (96%) were TNM stage T1 or T2 [9].
On mammography, the presence of mucin translates into a relatively well-defined, low-density lobular mass. Occasionally, they may have partially faded or obscured margins. Up to 20% of lesions may be occult on mammography.
Calcification may be rare in pure mucinous types. On ultrasonography, mucinous carcinomas often show mixed echogenicity with mixed solid and cystic components, distal enhancement and microlobulated margins are commonly found in mucinous carcinomas. A mixed mucinous carcinoma tends to be more hypoechoic. On MRI, they are one of the few cancers to have very high signal intensity on T2-weighted images involving the mucinous component, compared with other malignant breast tumors [10].
Lymph node metastases in pure colloid carcinomas of the breast are their frequency increases with tumor size. Immunohistochemical studies of hormone receptors for estrogen and progesterone have often revealed a strong presence, particularly of estrogen (91% of cases) [11].
Treatment is based on surgery with or without adjuvant chemotherapy and hormone therapy. Conservative surgical treatment (lumpectomy) is recommended for T1 and small T2 cases, followed by radiotherapy [12]. Partial and accelerated irradiation of the breast is currently the most recommended after conservative surgery [3]. Exclusive radiotherapy may be attempted in inoperable forms for local or general reasons [12]. Poortmans [12] reported a 70% reduction in the risk of locoregional recurrence in patients treated with irradiation, irrespective of age, tumour characteristics and systemic administration of treatment.
Most authors agree on the favorable evolution of pure colloid carcinoma compared to other forms of breast malignancy, in particular ductal carcinoma which is by far the most common histological type [11]. The onset of metastases in pure colloid carcinomas is delayed late. The average time to metastasis is ten years [13].
Conclusion
The distinction between pure and mixed colloid carcinoma is an important one, as the therapeutic attitude and prognostic impact depend on it. The prognosis of the mixed form, which is similar to that of infiltrating ductal carcinomas, is poorer than that of the pure form. Overall survival of women with mucinous carcinoma is better than that of infiltrating carcinoma, and surgery remains the best 1st-line therapy.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018; 68(6):394-424. [PubMed.]
- Liu H, Tan H, Cheng Y, Zhang X, Gu Y, Peng W. Imaging findings in mucinous breast carcinoma and correlating factors. Eur J Radiol. 2011;80(3):706-12. [PubMed.]
- Ishikawa T, Hamaguchi Y, Ichikawa Y, Shimura M, Kawano N, Nakatani Y, et al. Locally advanced mucinous carcinoma of the breast with sudden growth acceleration: a case report. Jpn J Clin Oncol. 2002;32:64-7. [PubMed.]
- Komenaka IK, El-tamer MB, Troxel A, Hamele-bena D, Joseph LA, Horowitz E, et al. Pure mucinous carcinoma of the breast. Am J Surg. 2004;187:528-32. [PubMed.]
- Fink C, Lüdemann H, Wasser K, Delorme S. Incidental finding of a mucinous carcinoma of the breast by dynamic MRI in a patient with a history of breast trauma. Clin Imaging. 2002;26:254-7. [PubMed.]
- Liu H, Tan H, Cheng Y, Zhang X, Gu Y, Peng W. Imaging findings in mucinous breast carcinoma and correlating factors. Eur J Radiol. 2011;80:706-12. [PubMed.]
- Kuhl CK. MRI of breast tumors. Eur Radiol. 2000;10:46-58. [PubMed.]
- Nowak H, Mignot L, Roquancourtde A, Pierre TF, Corin A. Carcinomes colloïdes muqueux du sein. À propos de neuf cas suivis au centre des maladies du sein de l’hôpital Saint-Louis. Gynecology. 2013;34:431-4. [Ref.]
- Fentiman IS, Millis RR, Smith P, Ellul JPM, Lampejo O. Mucoid breast carcinoma: histology and prognosis. Br J Cancer. 2007;75:1061-5. [PubMed.]
- Macbain M, Radswiki R, et al. Mucus carcinoma of the breast. [Ref.]
- Komenaka IK, El-tamer MB, Troxel A, Hamele-bena D, Joseph LA, Horowitz E, et al. Pure mucinous carcinoma of the breast. Am J Surg. 2004;187:528-32. [PubMed.]
- Ucla L, Fenton J, Mathieu G, Vilcoq J, Bateini JP. Cancers colloïdes du sein. Intérêt de la radiothérapie. Série de 138 cas traités à l’institut Curie. Bull Cancer. 1998;75:783-7. [Ref.]
- Komenaka IK, El-tamer MB, Troxel A, Hamele-bena D, Joseph LA, et al. Pure mucinous carcinoma of the breast. Am J Surg. 2004;187:528-32. [PubMed.]