>Corresponding Author : Shahid Aziz
>Article Type : Case Report
>Volume : 4 | Issue : 5
>Received Date : 04 March, 2024
>Accepted Date : 16 March, 2024
>Published Date : 18 March, 2024
>DOI : https://doi.org/10.54289/JCRMH2400123
>Citation : Imran M, Ali M, Aziz S, Ahmad B, May FEB, et al. (2024) Successful Correction of the Misdiagnosis of a Primary Liver Neuroendocrine Tumour with a Combination of Immunohistochemical and Radiological Assessments: Report of a Rare Case from Pakistan. J Case Rep Med Hist 4(5): doi https://doi.org/10.54289/JCRMH2400123
>Copyright : © 2024 Imran M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Patients Diagnostic Lab, Pakistan Institute of Nuclear Science and Technology, Islamabad, Pakistan
2Department of Biological Sciences, Islamic International University, Islamabad, Pakistan
3Department of Gastroenterology, Pakistan Institute of Medical Sciences, Islamabad, Pakistan
4Institute of Allied Health Sciences, Wah Medical College, National University of Medical Sciences, Rawalpindi, Pakistan
5Department of Pathology, Northern Institute for Cancer Research, Newcastle University, United Kingdom
6National Institute for Health Research, Southampton Centre for Biomedical Research Mass Spectrometry Lab, Southampton General Hospital, Southampton, United Kingdom
7School of Human Development and Health, Faculty of Medicine, University of Southampton, United Kingdom
*Corresponding author: Shahid Aziz, Institute of Allied Health Sciences, Wah Medical College, National University of Medical Sciences, Rawalpindi, Pakistan
Abstract
Human neuroendocrine tumours arise in different organs including most frequently in the gall bladder, kidneys, and ovaries or testicles. Liver neuroendocrine tumours are rarer types that grow slowly and arise from neuroendocrine cells. A 50-year-old woman with a history of type 1 diabetes mellitus and hypertension presented with severe abdominal pain, fever, pallor and jaundice over two days. She had not history of cancer. Initially, she was treated medically with Ciproxin, Entamizole, Zentel, and Nospa, but her symptoms were not resolved, and she was referred for gastroduodenal endoscopy, radiological and blood tests. The gastroduodenal endoscopic evaluation revealed severe gastritis with mild erythema in the corpus and antrum of the stomach with no evidence of esophagitis or duodenitis. At the time of initial diagnosis, the patient was asymptomatic for a liver neuroendocrine tumour as she did not have anemia, weight loss, abdominal distension, severe abdominal pain, or a palpable right upper quadrant mass.
The radiological investigations, abdominal and pelvic ultrasound and computed tomography scan, detected hepatomegaly with a mass of an internal necrotic area in left lobe and smaller lesions in the right lobe. Liver core biopsies taken from the left lobe contained hepatocellular carcinoma. After a hepatectomy of the left lobe, the patient was stable and managed with intravenous fluid, analgesics, antibiotics, and antiemetic drugs and was discharged. Hepatocellular carcinoma was diagnosed in the resection specimen. The resected tumour tissue was analysed through immunohistochemistry for presence of Sheppard, arginase, Cam5.2 and CDX-2, heppar, arginase, PAX8, Albumin ISH, CK7, CK20 and INSM1, synaptophysin, chromogranin, Ki67, Synaptophysin, Cytokeratin AE1/AE3, synaptophysin, CD56, and Glypican-3. Consequently, liver neuroendocrine cancer was diagnosed. The patient was treated with Sandostatin for 7 days to treat the neuroendocrine tumour. Following a left hepatectomy, post-surgery endoscopic retrograde cholangiopancreatography (ERCP) showed normal ampullary mucosa and successful surgical biliary drainage was observed for almost 45 days with bile drain effluent between 20-230 ml. In this case, the differentiation of this liver neuroendocrine tumour from hepatocellular carcinoma, hyper vascular hepatic lesions based upon detection of chromogranin, CK7, CK20, HepPar1, Ki67 allowed appropriate clinical management of the patient. During radiological analysis, it was confirmed that the liver mass was not metastasized from the primary tumour of the stomach or colon.
Abbreviations: ERCP: Endoscopic Retrograde Cholangiopancreatography NET: Neuroendocrine Tumours CEA: Carcinoembryonic Antigen AFP: Alpha-Fetoprotein CA: Cancer Antigen, CBD: Common Bile Duct HCC: Hepatocellular Carcinoma SPECT: Single Photon Emission Computed Tomography WHO: World Health Organization MRI: Magnetic Resonance Imaging PET: Positron Emitting Tomography
Introduction
Neuroendocrine tumours (NETs) have a prevalence rate of 1-2% among all gastrointestinal tract tumours. They are higher in the trachea bronchopulmonary system and pancreas [1]. A study that described NETs in 1958 [2] reported the liver as the most common site of metastatic spread for these tumours [3]. While, among all cases of NETs, primary liver NETs are extremely rare 0.3% [4] and are diagnosed mostly in females. The clinical diagnosis of primary liver NETs is complicated due to non-specific diagnostic investigations and vascular hepatic imaging mimics [5].
Patients mostly remain asymptomatic, while in some cases clinical symptoms such as fatigue, right-sided abdominal pain, obstructive jaundice, weight loss, flushing of the skin, palpitations, and diarrhea have been reported. Moreover, various research studies have reported non-specific but normal levels of tumour markers including carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), cancer antigen (CA) 125, and CA 19-9 [3,6-8]. In this case report, different specific antibodies and radiological tests were used to detect liver NETs.
Case Presentation
A 50-year-old female patient with a history of type 1 diabetes mellitus and hypertension presented to her primary health care practitioner, with severe abdominal pain of two days duration accompanied by pallor and jaundice but without a fever. She was treated with different drugs such as Rabecid, Nospa, Motilium, Ciproxin, Entamizole DS, and Zentel and but her symptoms were not resolved. The patient was referred to the gastroenterologist and admitted to the Department of Gastroenterology, Pakistan Institute of Medical Sciences, Islamabad, Pakistan. Based on clinical complications, some routine tests such as complete blood count, C reactive protein level, renal and liver function tests, serum lipase and amylase, lipid profile, blood sugar (random and fasting), routine urine analysis (Table 1), radiological investigations (ultrasound abdomen and pelvis) and upper gastroduodenal endoscopic and colonoscopic procedures were performed to investigate the cause of her abdominal pain. While her diagnosis was awaited, the patient was treated with various drugs including Rabeprazole, Drotavin, Motilium, Ciproxin, Albenza, and Ornidazole to relieve her pain.
The gastroduodenal endoscopy showed severe gastritis with mild erythema in the corpus and antrum of the stomach with no evidence of esophagitis and duodenitis. Moreover, the colonoscopic procedure revealed a small rectal polyp, and multiple biopsies were taken for histopathological examination, that revealed hyperplastic changes with no evidence of dysplasia. The ultrasound of the abdomen and pelvis showed an enlarged liver measuring 187 mm in size toward the craniocaudal direction and the contour was smooth and moved freely with respiration having normal echogenicity and echotexture. A large non-compressible solid mass measuring 105×153 mm in size was present in the left lobe with multiple small cystic areas occupying the right lobe of the liver surrounded by unremarkable parenchyma in the epigastric region. The partially contracted gall bladder was normal in size. The common bile duct (CBD) was not dilated and the portal vein was normal. Moreover, the splenic span was 111 mm, which exhibited smooth and homogenous parenchymal echotexture with no evidence of focal or diffuse lesion varicose or enlarged lymph nodes at the splenic hilum. Both kidneys were normal, with no stones or cysts, but perinephric abnormalities were noted. Additionally, the uterus was not dilated and the urinary bladder was normal having no evidence of adenopathy in the abdomen.
The abdominal CT scan with oral and IV contrast indicated an enlarged liver of 18.7 cm with multiple low-density areas, the left lobe measured 13.6×10.6×11 cm along and there were other smaller lesions mainly in segments V, VII, and VIII. These lesions showed persistent perilesional enhancement mainly in the proto-venous and late phases with multiple separations inside. The inferior vena cava and portal vein were compressed. The gall bladder was normal and the pancreas had normal texture and density with compression from the anterior aspect. The spleen was 11.8×5.3×6.7 cm with a splenic index of 420. Both kidneys were normal. Mild degenerative changes were noted in the spine and multiple enhancing fluid density areas were observed in a large focus with septation in the left lobe. The CT scan also showed that the left lobe mass had an internal necrotic area. A hydatid cyst was suspected but serology proved negative. Moreover, a non-contrast CT scan of the chest showed a few calcified and non-calcified mediastinal lymph nodes, osseous degenerative changes, and an upper abdomen with a large mass in the left lobe of the liver and a few hypodense cystic lesions in both lobes.
Based on these findings, ultrasound-guided liver mass biopsies were taken for histopathological examination to investigate possible malignancy. Abundant cells with eosinophilic cytoplasmic and hyper-chromic nuclei were observed. HepPar1 and arginase-1 were detected through immunohistochemistry whereas CK7 and CK20 were absent. The histopathological report was of a moderately differentiated hepatocellular carcinoma (HCC).
Table 1: Differential pathological tests for clinical correlation
| Blood Tests | Patient Values | Reference Values |
|---|---|---|
| Total Leukocytes Count | 8740/mm3 | 4000-10,000/mm3 |
| Hemoglobin | 13.3 g/dl | 11.5-16.5 g/dl |
| Platelets count | 312,000/ mm3 | 1,50,000-4,50,000 /mm3 |
| Total RBC count | 3.01×1012 | 3.8-5.8×1012 |
| Hematocrit | 40 l/l | 41-45 l/l |
| MCV | 73 fl | 82-98 fl |
| MCH | 24.3 pg | 27-31 pg |
| MCHC | 33.5 g/dl | 32-36 g/dl |
| RDW-CV | 15 % | 11-16% |
| Neutrophil | 60 % | 40-75% |
| Lymphocyte | 29% | 15-45% |
| Monocyte | 9% | 2-12% |
| Eosinophil | 2% | 2-6% |
| C-reactive protein | 14.4 mg/dl | >5.0 mg/dl positive |
| Serum Amylase | 66U/l | 28-100U/l |
| Serum Lipase | 86 U/l | 13-60 U/l |
| Blood Glucose (Fasting) | 238 mg/dl | 70-110mg/dl |
| Glycosylated HBA1C | 9.4% | < 7.0% goal for diabetes |
| Serum Cholesterol | 173 mg/dl | <200 mg/dl |
| HDL Cholesterol | 51 mg/dl | 40-60 mg/dl |
| LDL Cholesterol | 111 mg/dl | 100-130 mg/dl |
| Triglyceride | 100 mg/dl | < 150 mg/dl |
| VLDL Calculated | 11 mg/dl | 5-40 mg/dl |
| Gamma GT | 88 IU/l | 9-36 IU/l |
| Total Bilirubin | 0.6 mg/dl | 0.2-1.0 mg/dl |
| ALT | 63 U/L | 5-50 U/L |
| ALP | 174 U/L | 35-104 U/L |
| AST | 54 U/L | 10-40 U/l |
| Alpha Fetoprotein Protein | 4.4 ng/ml | 0-7.0 ng/ml |
| CEA level | 6.25 ng/ml | 5.0 ng/ml |
| CA-125 | 10 IU/ml | <20.0 IU/ml |
| CA 19-9 | <2. 0 IU/ml | 37 IU/ml |
| Protein Induced by Vitamin K Absence -II (PIVKA-II) | 45.6 mAU/ml | <40 mAU/ml |
| Serum Magnesium level | 1.9 mg/dl | 1.6-2.6 mg/dl |
| Serum Sodium | 140 mmol/l | 136-145 mmol/l |
| Potassium | 4.9 mmol/l | 3.5-5.1 mmol/l |
| Chloride | 105 mmol/l | 98-107 mmol/l |
| Bicarbonate | 24.5 mmol/l | 20-31mmol/l |
| Gamma GT | 108 IU/L | < 38 IU/L |
| Calcium | 10.0 mg/dl | 8.6-10.2 mg/dl |
| Phosphorus | 3.7 mg/dl | 2.5- 4.5 mg/dl |
| Urine Protein | +++ | |
| Urine Glucose | + | |
| Entamoeba histolytica Ab | Negative | |
| Echinococcus IgG Ab | Negative | |
A TC.99 Sandostatin scan, which allows detection of carcinoid tumours including NETs was performed, with whole body images acquired with a dual head gamma camera within one to four hours. Single photon emission computed tomography (SPECT) imaging of the abdomen revealed a large, solid tracer avid lesion present in the left lobe of the liver causing compression of nearby abdominal structures. In comparison, the smaller lesion was also noted in the right lobe of the liver which showed no or negligible tracer uptake. Tracer avid lesions were not detected elsewhere in the body that could have been a primary lesion. Normal radiotracer distribution was observed in the spleen, gall bladder, bowel, kidneys, and urinary bladder.
After receipt of the SPECT scan report, the treating physician decided to review the case because the imaging findings did not agree with the diagnosis of HCC. The case was reviewed via histology support in Pakistan, and a second opinion on the liver core biopsies was again sought from the histopathologist. The diagnosis of HCC based upon the tumour cells having abundant pink cytoplasm; with focal but strongly positive expression of Hep Par1 and arginase, along with presence of hepatoblastoma, as well as hepatic adenoma indicate HCC. Contemporaneously immunohistochemical analyses demonstrated presence of synaptophysin and chromogranin which are produced by neuroendocrine cells, while CK-7 and CK-20 were not detected. Based upon these findings, a primary HCC with neuroendocrine differentiation was reported. A second opinion was sought from a separate team of histopathologists in a different hospital of Pakistan. It reported that the liver tissue contained cores of liver parenchyma infiltrated by a lesion composed of nests and sheets of cells with mild nuclear atypia and abundant pink cytoplasm. Immunohistochemical analyses detected epithelial cytokeratin with the pan cytokeratin AE1/AE3 antibodies alongside synaptophysin (Figure 1) but not cytokeratin 7 or cytokeratin 20, which are found in lung, breast, ovarian, endometrial, gastrointestinal and uroepithelial lesions respectively. CD56, glypican-3 or Hep-Par1. Ki-67 was detected in less than 2% of the malignant cells. Consequently, the final diagnosis was of a well-differentiated NET, WHO grades I.
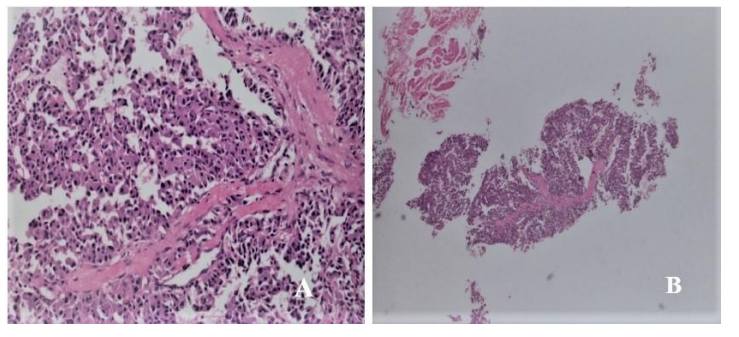
Figure 1: Histopathological examinations of the core liver biopsy specimens revealed hepatocellular carcinoma: positive expression of cytokeratin AE1/AE3 antibodies (A: H&E, 100×) and synaptophysin (B: H&E, 10×).
The physician decided to have the diagnosis of NET assessed at an international center. The Johns Hopkins Medicine, 401 N, Broadway, Baltimore was selected for this purpose. At this institute, various NET markers were analyzed through immunohistochemistry. Cam 5.2, CDX-2 and Hep-Par1 were detected focally while arginase-1, PAX8, albumin ISH, CK7, CK20, and INSM1 were absent (Figure 2). The diagnosis was of a well-differentiated NET, WHO Grade 1. Subsequent to the clinical diagnosis of a NET, the presence of additional neuroendocrine biomarkers was assessed. Synaptophysin and chromogranin were detected diffusely and focally within the malignant cells while, Ki-67 detection indicated a proliferative index of less than 2%. These findings supported the diagnosis of a primary liver NET.
After diagnosis, the patient was treated with Sandostatin 100 mg which controls the growth of some advanced NETs for 7 days. After 2 months, a hepatectomy of the left lobe and microwave ablation of the right lobe lesion was performed. Intraoperative findings showed a large tumour in the left lobe of the liver while four other lesions were also identified in the right lobe of the liver segments 4b, 7, and 8. These smaller lesions were treated with microwave ablation. The patient was vitally stable, kept nil per oral, and managed with intravenous fluid, analgesics, antibiotics, and antiemetic drugs. After one week the patient was discharged with the following medication: Nexium 20 mg/od, Artifen and Zivus/bid, Psyllium hydrophilic mucilloid 100 gm/conc (20gm/od), Cremaffin 120ml (30ml/od) and Caricef (400 mg/od) for 7 days.
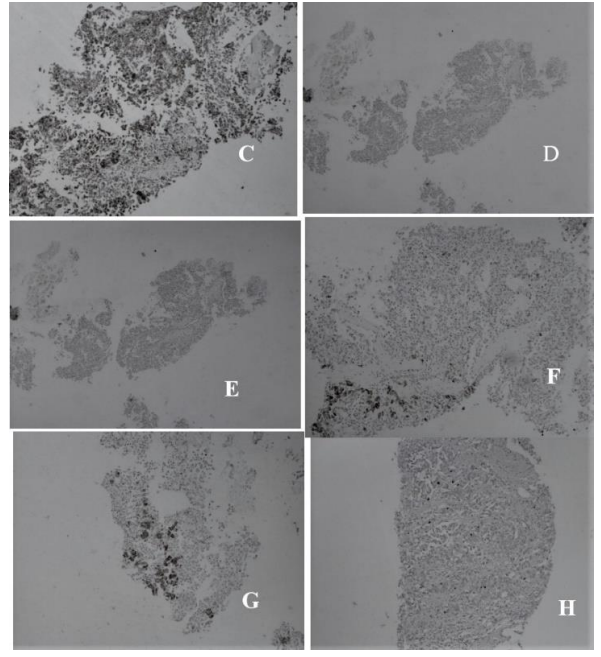
Figure 2: Immunohistochemical analyses for identification and differentiation of primary liver NET; C: Chromogranin +ve, 100×, D: CK-7 –ve, 10×, E: CK20 –ve, 10×, F: Hep-Par1 +ve, 100×, G: Hep-Par1 +ve, 10×, H: Ki-67 value < 2%, 100×.
Histopathological examination of the resected left lobe identified three brown nodules with the largest measuring 1.5×1.5×1.5 cm. Multiple tissue regions were taken for histopathological examination such as the largest and smaller nodules from the liver, tumour with resection margin, random sections of the tumour, and falciform ligament. Histopathological analysis showed a similar morphological neoplastic lesion composed of a sheet and aggregate of oval to polygonal-shaped cells such as eosinophilic cytoplasm, nuclei, and chromatin. The nuclei showed a moderate to marked degree of pleomorphism with variable prominent nucleoli. A few of the neoplastic cells appear large with more than one nuclei. Moreover, a scattered mitotic figure was also noted in an adjacent area showing extensive necrosis while the section from the falciform ligament was negative for malignancy. Immunohistochemical analyses, indicated the presence of cytokeratin AE1/AE3 antibodies, synaptophysin, and CD56 while TTF-1, chromogranin, and CDX2 were absent and Ki-67 was detected in less than 2 % of cells indicative of a low mitotic index.
The abdominal sonography of the liver showed moderate fatty parenchymal echotexture with a smooth outline, no focal defect of the intrahepatic biliary channel, and dilation of hepatic veins. The post-lobectomy of the left liver lobe was absent and a small cyst formation was observed in the anterior segment of the right lobe measuring 3×3×3 cm having an approximate volume of 14 ml and in situ, the drainage tube was noted. Moreover, the porto-splenic mesenteric venous was of normal caliber and the gall bladder showed cystectomy. The common bile duct (CBD) was not diluted, the pancreas had a smooth outline, normal in size and parenchymal echotexture, no focal mass was observed and the tail was obscured by gastric gaseous reverberation while the pancreatic duct was not detected. Furthermore, the spleen was not enlarged and no focal defect was detected. The size of the right kidney was normal with a smooth outline having no evidence of parenchymal pathology. Additionally, normal cortical thickness and no stone/mass hydronephrosis were noted. The endoscopic retrograde cholangiopancreatography (ERCP) procedure showed normal ampullary mucosa.
Incidentally, the guide wire went into the pancreatic duct which was pulled back immediately and selective CBD cannulation was done after a few attempts with slight difficulty using a cannulotome over 0.025 cm guide wire, and stranded sphincterotomy was performed with sphincterotome. The cholangiogram showed normal CBD and trickling of dye was observed from the right biliary system and single plastic CBD stent 12 cm × 10 Fr was placed. Interestingly, good bile flow was noticed through the stent. Further, normal caliber cyst and right hepatic ducts were also noted on the cholangiogram. Trickling of dye was noticed at one of the branches of the right hepatic duct indicating leakage (Figure 3).
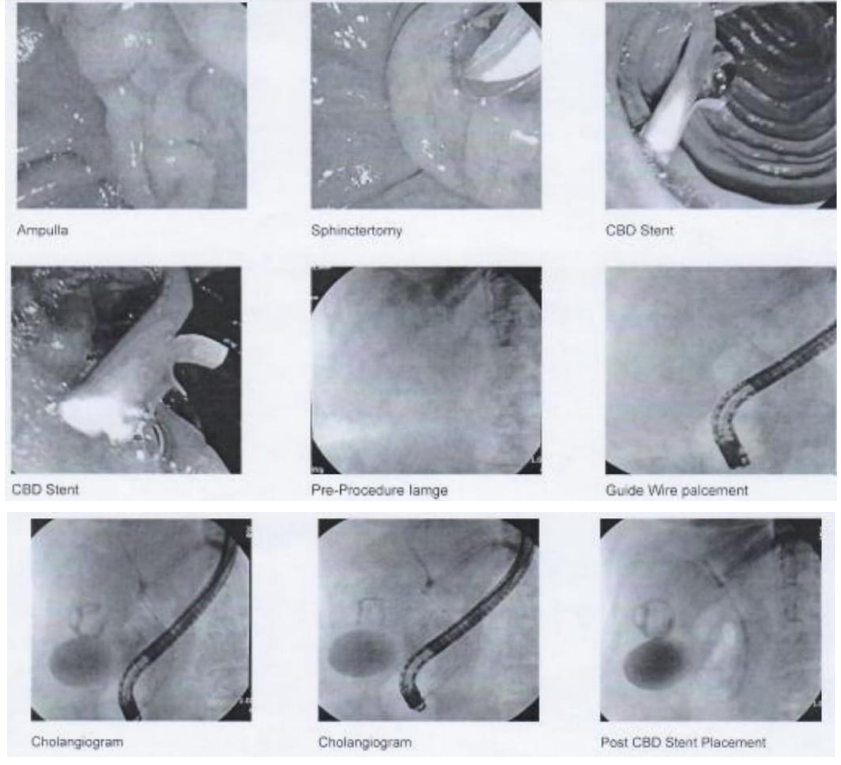
Figure 3: The patient underwent post-surgical retrograde cholangiopancreatography (ERCP) procedure after overnight fasting. The ERCP showed normal ampullary mucosa, sphincterotomy, CBD stent, pre-procedure image, guide wire placement, cholangiogram, and post CBD stent placement.
The dynamic contrast-enhanced CT of the chest and liver showed no segmental collapse, consolidation, pleural effusion, or pneumothorax. Minimal atelectasis was observed in the right middle lobe and a calcified sub-pleural nodule was seen in the right lung. Moreover, a few calcified/non-calcified hilar and mediastinal lymph nodes were observed and a prominent lymph node measuring 1.2 cm in the short axis was noted in the left axillary tail. Additionally, a few prominent pericardial lymph nodes were observed up to a maximum of 8.8 mm while the heart was normal in size with no pericardial effusion. A small hypodense nodule was visible in the left lobe of the thyroid gland and fibro-fatty breast parenchyma was noted bilaterally without any definite mass lesion.
The surgical staples were not visualized at the left lobe of the liver hilum, in the middle of the left portal and hepatic veins. Importantly, a hyperdense drain was observed along the resected margin of the liver with strand changes in the surgical bed. The CBD stent was noted in situ with the proximal tip in one of the right hepatic ducts and the distal tip at the ampulla/second part of the duodenum. The minimal intrahepatic biliary prominence involving segment VII was observed with a surgical scar with stranding and mild fluid in the anterior abdominal wall in the midline and right hypochondrium. The perfusion-related changes were observed due to heterogeneous and irregular nodular arterial enhancement of liver parenchyma involving segments VII. Two well-defined hypodense lesions in the liver involving segments IVA, V, and VII showed an internal hyperdense component with mild peripheral enhancement in the delayed phase.
Multiple prominent lymph nodes were observed in the upper abdomen in perigastric and periceliac regions with few prominent mesenteric nodes having no ascites. The spleen and pancreas were normal. Moreover, bilateral adrenal glands were normally visualized and the gallbladder was partially distended with tiny air locules within its lumen, likely due to stent placement. Additionally, tiny hypodensities were noted in bilateral kidneys which were too small to characterize. And no abnormal small or large bowel dilatation was observed while the urinary bladder was thick-walled and almost collapsed. Uterus and adnexa showed no gross abnormality. No significant osseous abnormality was seen and, multilevel degenerative changes were also noted with minimal anterolisthesis in L3 over L4 and L5 over S1. Interestingly, the successful surgical procedure of biliary drainage was observed for almost 45 days with bile drain effluent between 20-230 ml (Figure 4).
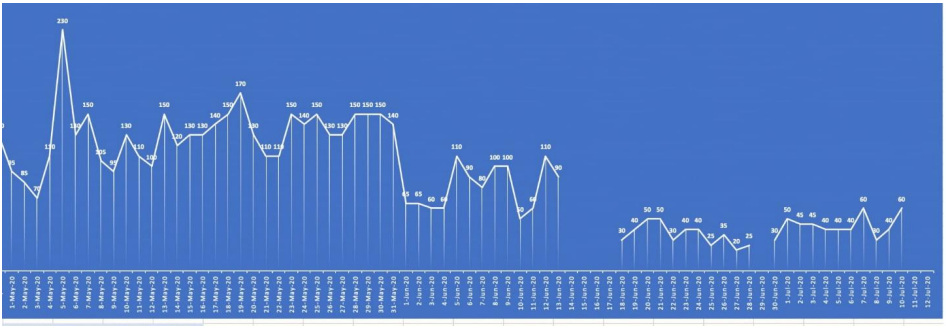
Figure 4: Assessment of post-surgical bile drain effluent: 20-230 ml bile drain effluent was observed during biliary drainage for almost 45 days.
Discussion
Neuroendocrine tumours (NETs) are extremely rare, with fewer than 200 cases reported since Edmondson first described the disease in 1958. There have been relatively few studies about its symptoms, treatment and outcome. NETs are derived mainly from the neuroectodermal cells which are found throughout the human body [7]. In a study in the United States, an incidence rate of 6.25 cases/ 100, 000 of NETs was reported [9]. It has been observed that between 54-90% of all NETs arise within the gastrointestinal tract and that amongst these cases; primary liver NETs are exceptionally rare. They account for 0.3% of cases of all NETs [10].
So far, no classification system has been established for grading primary liver NETs except, the 2010 World Health Organization (WHO) classification of the gastroenteropancreatic neuroendocrine tumour grading system. Based on this grading system, tumours are classified as well differentiated tumours having low grade malignancy (grade I), well differentiated tumours with intermediate grade neoplasms (grade II) and poorly differentiated or high grade neoplasms (grade III), [11]. This classification system is useful to assess the prognosis and malignant potential of the primary liver NETs [12]. In this reported case, the patient had a diagnosis of a well differentiated NETs WHO grade I.
Liver could be the location of metastatic NETs, for example metastatic carcinoid tumour. Therefore, it was particularly important to determine if the lesion was a primary NET or a secondary that had metastasized from a metastatic primary NET located elsewhere in the body. Primary liver NETs grow at a slower rate than other NETs and are, mostly case diagnosed at an advanced stage. Their low mitotic index helps to distinguish them from other NETs or from secondary tumours that have metastasized from other primary NETs [13]. In most cases, the primary liver NETs are discovered incidentally which appear as endocrinologically silent hepatic masses and amongst patients only 6.8% have classic carcinoid symptoms including abdominal pain, skin flushing, and diarrhea [3].
The clinical presentation of primary liver NETs can be compared with a hepatic metastatic spread from extrahepatic neuroendocrine tumours which are usually associated with typical carcinoid syndrome. The primary liver NETs are identified due to their symptoms related to special effects of mass on the liver and their nearby areas including palpable right upper quadrant mass, vague pain, abdominal distension and jaundice. In a comprehensive review, a total of 124 cases of primary liver NETs were diagnosed at the mean age of 51.9 years with no obvious sex predilection reported in males 49.2% and females 50.8% [3]. While in another study, primary liver NETs were reported more 58.5% in females as compared to males [6], so do in our case report.
Additionally, most NETs (76.6%) were reported as solitary nevertheless could also be multicentric along with right lobar preference (46.8%) [6]. In our case report, the patient was a 50-year-old female who had general symptoms of a liver tumour, which was located in both right and left lobes of the liver and was diagnosed after her physician requested a histology review of her case, because the patient’s symptoms did not correlate well with the original diagnosis. The HCC less likely to be diagnosed on CT scan as compared to blood tests and histopathological examination which can direct the clinicians in true diagnosis of HCC [14]. The same pattern was observed in our case report in which the initial histopathological diagnosis of HCC did not agree well with the radiological findings including those from the CT scan.
The identification of primary liver NETs mostly starts from the pre-operative stage and ends in post-surgical stage with continuous follow-ups for extra hepatic tumours [3]. In the pre-operative various research studies, primary liver NETs are mostly misdiagnosed as hepatic cellular carcinoma or cholangiocarcinoma. The radiological features of primary liver NETs can have higher variation with some of the lesions appearing as cyst, solid, diffuse, or well circumscribed margins [15]. In our case, solid tumour lesions were identified on the left and right sides of the liver.
The hepatectomy was carried out from the left side of the liver while right sided lesions were treated with microwave ablation. The primary liver NET ironically have higher blood supply in hepatic arteries, while large lesions in the arterial phase also have increased blood supply [16]. Both techniques were used in our case. The HCC have distinctive outlines of severe arterial enhancement along with failure in the portal and delayed phases which easily creates confusion in the diagnosis of primary liver NETs. In our case report of primary liver NET, the patient had mild peripheral enhancement in the delayed phase.
Multiple studies have reported pre-operative identification for primary liver NETs with the help of needle biopsy specimens with less diagnostic accuracy [17]. This approach yields a less accurate diagnosis which leads to misdiagnosis of primary liver NETs as HCC or cholangiocarcinoma [18]. The primary liver NET may be misdiagnosed during histopathological examination of liver biopsy specimens while in comparison postoperative histopathological and immunohistochemical examinations help out in accurate diagnosis of primary liver NETs [3]. The same approach has been used in this case report by taking core liver biopsies for initial diagnosis which revealed HCC. After hepatectomy and analysis with antibodies such as Chromogranin, Hep-Par1 and H: Ki-67 which changed the initial diagnosis of HCC into a primary liver NETs.
The radiological results are alike for both of the primary and metastatic NETs. Additionally, the pathological characteristics of primary liver NETs are hard to differentiate from hepatic metastases [19]. Thus vigilant examinations are necessary to exclude the existence of extrahepatic NETs. These investigations include CT scan, magnetic resonance imaging (MRI), somatostatin scintigraphy, positron emitting tomography (PET) scan, bronchoscopy plus operative examination, gastroduodenal and colonoscopic procedures.
After the primary tumour is still considered to be a liver NET even after detailed examinations, reevaluation with MRI, CT and PET scans are valuable to identify extrahepatic tumours which may have initially not been diagnosed [20]. Infrequently, the patients are traced with close follow up after surgical procedures for the final identification of primary liver NETs [21]. In our case, various radiological tests such as CT and TC.99 Sandostatin scans were performed along with endoscopic and colonoscopic procedures, histopathological and immunohistochemical examinations for final diagnosis of a primary liver NET during hospital admission.
The macroscopic examination of primary liver NETs revealed them as gray-yellow along with well-defined mass with various uneven hemorrhagic lesions or else with the cystic areas [22], having a size from 3.2 to 18 cm [18]. While in our case, the size of the primary liver NET was 11×7×3 cm with large encapsulated cystic tumours measured at 14.5×10.5×9.5 cm during gross examinations. The routine histopathological examinations with hematoxylin eosin staining showed neoplastic lesions composed of sheets and aggregates of an oval to polygonal shaped cells such as eosinophilic cytoplasm, nuclei and chromatin which are not specific for NETs which is only helpful for tumour classification. Three protein biomarkers including chromogranin A, synaptophysin and neuron specific enolase are used to diagnose NETs. It is in agreement with these findings that the tumour in our case was also immunoreactive for chromogranin A and synaptophysin.
Moreover, no standard treatment guidelines for primary liver NETs have been proven to be effective except for hepatectomy [23]. The primary liver NETs are related with a resectability rate of 70% with five years survival rate afterward hepatectomy of 78% cases [9]. While another study reported that the extent of disease and surgery type does not have a specific effect on the survival rate of patients with primary liver NETs. For treatment of patients with the un-resectable disease, multiple initial treatment options including are available systemic 5 fluorouracil [24] hepatic artery embolization [25] and octreotide therapy [26]. Nevertheless, research data on these treatment options are inadequate. Presently, liver transplantation has been recommended to be the only treatment option in particular patients diagnosed with various lesions or compromised liver function [27].
Primary liver NETs are asymptomatic and extremely rare compared to other NETs. They are relatively challenging to differentiate from other liver tumours including HCC and cholangiocarcinoma, based upon medical imaging. The primary liver NETs should be doubted in the patients even having no long lasting liver disease with normal serum values of alpha fetoprotein along with solitary hyper-vascular tumours in other radiological imaging investigation studies. The differentiation of primary liver NET from other extra hepatic masses is necessary. The diagnosis of a primary liver NET can be determined with thorough clinical assessment to eliminate another possibility that the lesion has arisen from another primary organ.
Conclusion
We described a rare case of a primary liver NET, which was initially considered to be a moderately differentiated HCC. The immunohistochemical analyses helped to differentiate between neuroendocrine and other extra hepatic tumours based upon detection of synaptophysin and chromogranin which are present in neuroendocrine cells. Additionally, cam 5.2 and CDX-2 were detected easily and appeared to be abundant in the malignant cells whereas CD56, glypican-3, Hep-Par1, CK-7, CK-20 arginase-1, PAX8, albumin ISH (in situ hybridization), and Insulinoma associated protein 1 (INSM1) were not detected. Ki-67 was present in less than 2% of the malignant cells indicative of a low mitotic index. Immunohistochemical analyses to assess presence of these proteins in recommended assisting in the identification and differentiation of NETs, especially primary liver NETs. Due to their uncommon presentation, it is important to exclude other possible etiologies before making a definitive diagnosis. Radiological investigations, such as Sandostatin scan is sensitive for assessment of metastatic NETs. Histological assessment of liver tissue whether obtained as a biopsy or at resection is the only accurate diagnosis. Complete surgical resection of the tumours mass is the only option that will provide the best chance of recovery.
Ethics approval and consent to participate: This case report was a part of a PhD research study and the ethical approvals were obtained from Pakistan Institute of Nuclear Science and Technology (PINST/DC-31/2021), Islamic International University (108-FBAS/PhDBT/F-19) and Pakistan Institute of Medial Sciences (F.1-1/2015/ERB/SZABMU). Informed written consent was obtained from the patient.
Competing interests: The authors declare that they have no competing interests.
Funding: This case was part of a PhD research study financially supported by the Pakistan Science Foundation (Project # PSF/Res/C-PINSTECH/Med531).
References
- Modlin IM, Lye KD and Kidd M. (2003) A 5‐decade analysis of 13715 carcinoid tumors Cancer: Interdisciplinary International Journal of the American Cancer Society. 97(4): 934-959. [PubMed.]
- Edmondson HA. (1958) Tumors of the liver and intrahepatic bile ducts (Vol 25). Armed Forces Institute of Pathology. [Ref.]
- Quartey B. (2011) Primary hepatic neuroendocrine tumor: what do we know now? World journal of oncology. 2(5): 209. [Ref.]
- Modlin IM, Kidd M, Latich I, Zikusoka MN and Shapiro MD. (2005) Current status of gastrointestinal carcinoids. Gastroenterology. 128(6): 1717-1751. [PubMed.]
- Agha RA, Fowler AJ, Rammohan S, Barai I and Orgill DP. (2016) The PROCESS statement: preferred reporting of case series in surgery. Int J Surg. 36(Pt A): 319-323. [PubMed.]
- Lin CW, Lai CH, Hsu CC, Hsu CT, Hsieh PM, et al. (2009) Primary hepatic carcinoid tumor: a case report and review of the literature. Cases Journal. 2(1): 90. [Ref.]
- Song JE, Kim BS and Lee CH. (2016) Primary hepatic neuroendocrine tumor: a case report and literature review. World journal of clinical cases. 4(8): 243. [Ref.]
- Huang YQ, Xu F, Yang JM and Huang B. (2010) Primary hepatic neuroendocrine carcinoma: clinical analysis of 11 cases. Hepatobiliary Pancreat Dis Int. 9(1): 44-48. [PubMed.]
- Knox CD, Anderson CD, Lamps LW, Adkins RB and Pinson CW. (2003) Long-term survival after resection for primary hepatic carcinoid tumor. Annals of surgical oncology. 10(10): 1171-1175. [PubMed.]
- Camargo ÉS, Viveiros MDM, Corrêa Neto IJF, Robles L and Rezende MB. (2014) Primary hepatic carcinoid tumor: case report and literature review. Einstein (Sao Paulo). 12: 505-508. [PubMed.]
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV and Suster S. (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature grading and staging systems. Pancreas. 39(6): 707-712. [PubMed.]
- Zhao J, Yang B, Xu C, Zhang W S, Ji Y, et al. (2012) Study on clinicopathologic grading system and prognosis of primary hepatic neuroendocrine neoplasms Zhonghua bing li xue za zhi. Chinese journal of pathology. 41(2): 102-106. [PubMed.]
- Jia C, Zhang Y, Xu J and Sun K. (2012) Experience in primary hepatic neuroendocrine tumor. Turk J Gastroenterol. 23(5): 546-551. [PubMed.]
- Asafo-Agyei KO, Samant H. (2023) Hepatocellular Carcinoma. Treasure Island (FL). StatPearls Publishing. [Ref.]
- Kellock T, Tuong B, Harris AC and Yoshida E. (2014) Diagnostic imaging of primary hepatic neuroendocrine tumors: a case and discussion of the literature. Case Reports in Radiology 2014. [Ref.]
- Wang LX, Liu K, Lin GW, and Jiang T. (2015) Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 15(1): 1-7. [Ref.]
- Skagias L, Vasou O, Ntinis A, Kondi-Pafiti A, Koureas A, et al. (2010) Primary Hepatic Neuroendocrine Tumor with Exophytic. Growth Acta cytologica. 54(2): 202-204. [Ref.]
- Hwang S, Lee Y J, Lee S G, Kim CW, Kim KH, et al. (2008) Surgical treatment of primary neuroendocrine tumors of the liver. Journal of Gastrointestinal Surgery. 12(4): 725-730. [PubMed.]
- Baek SH, Yoon JH and Kim KW. (2013) Primary hepatic neuroendocrine tumor: gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging Acta radiologica short reports. 2(2): 1-5. [PubMed.]
- Landen S, Elens M, Vrancken C, Nuytens F, Meert T, et al. (2014) Giant hepatic carcinoid: a rare tumor with a favorable prognosis. Case Reports in Surgery 2014. [Ref.]
- Donadon M, Torzilli G, Palmisano, A Del Fabbro, D Panizzo, et al. (2006) Liver resection for primary hepatic neuroendocrine tumours: report of three cases and review of the literature. European Journal of Surgical Oncology (EJSO). 32(3): 325-328. [PubMed.]
- Shetty PK, Baliga SV, Balaiah K and Gnana P. (2010) Primary hepatic neuroendocrine tumor: an unusual cystic presentation. Indian Journal of Pathology and Microbiology. 53(4): 760. [PubMed.]
- Yalav O, Ülkü A, Akcam T, Demiryürek H and Doran F. (2012) Primary hepatic neuroendocrine tumor: five cases with different preoperative diagnoses. Turkish Journal of Gastroenterology. 23(3): 272-278. [PubMed.]
- Andreola S, Lombardi L, Audisio RA, Mazzaferro V, Doci R, et al. (1990) A clinicopathologic study of primary hepatic carcinoid tumors Cancer 65(5): 1211-1218. [PubMed.]
- Krishnamurthy SC, Dutta V, Pai SA, Kane SV, Jagannath P, et al. (1996) Primary carcinoid tumor of the liver: report of four resected cases including one with gastrin production. Journal of surgical oncology. 62(3): 218-221. [PubMed.]
- Wängberg B, Nilsson O, Johanson V, Kölby L, Forssell-Aronsson E, et al. (1997) Somatostatin receptors in the diagnosis and therapy of neuroendocrine tumors. The oncologist. 2(1): 50-58. [PubMed.]
- Fenwick SW, Wyatt JI, Toogood GJ and Lodge JPA. (2004) Hepatic resection and transplantation for primary carcinoid tumors of the liver. Annals of surgery. 239(2): 210. [PubMed.]