>Corresponding Author : Elisa Nuez-Zaragoza
>Article Type : Case Report
>Volume : 4 | Issue : 4
>Received Date : 14 Feb, 2024
>Accepted Date : 26 Feb, 2024
>Published Date : 29 Feb, 2024
>DOI : https://doi.org/10.54289/JCRMH2400120
>Citation : Nuez-Zaragoza E, Bhambi-Blanco I, Baena N, Perea G, Martínez M, et al. (2024) A Review of Three Cases of Primary Plasma Cell Leukemia: Incidental Finding Discovered by Blood Smear Revision. J Case Rep Med Hist 4(4): doi https://doi.org/10.54289/JCRMH2400120
>Copyright : © 2024 Nuez-Zaragoza E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Hematology Laboratory, Clinical Laboratories Department. Parc Taulí Hospital Universitari. Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA). Universitat Autònoma de Barcelona. Sabadell, Spain
2Genetics Laboratory, Clinical Laboratories Department. Parc Taulí Hospital Universitari. Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA). Universitat Autònoma de Barcelona. Sabadell, Spain
3Hematology Department. Parc Taulí Hospital Universitari. Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA). Universitat Autònoma de Barcelona. Sabadell, Spain
*Corresponding author: Elisa Nuez-Zaragoza, Hematology Laboratory, Clinical Laboratories Department. Parc Taulí Hospital Universitari. Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA). Universitat Autònoma de Barcelona. Sabadell, Spain
Abstract
Introduction: Plasma cell leukemia (PCL) is the most aggressive variant of the monoclonal gammopathies (MG). Its diagnosis is based on standard morphological examination of peripheral blood, by finding plasma cells with an atypical morphology. The International myeloma working group (IMWG) has updated the diagnostic criteria for PCL considering that patients with newly diagnosed MG with 5% or greater plasma cells in peripheral blood should be considered as PCL.
Case report: We present three cases of primary PCL that were admitted to the hospital due to the study of an anaemia and whose diagnosis was an incidental finding. All the patients had >5% of circulating plasma cells, whose clonality and aberrant phenotype was determined by peripheral and blood marrow flow cytometry immunophenotype and all of them had high-risk cytogenetics related with a poor and adverse outcomes of the disease.
Discussion: This cases highlight the importance of peripheral blood smear revision, because the identification of aberrant plasma cells leaded the diagnosis of the MG and the patients were able to start treatment as soon as possible. Carefully examination and interpretation of peripheral blood smear should be always performed, with special attention in patients with a suspicion of MG, because with the new IMWG definition, it is possible that this entity is underdiagnosed.
Keywords: Aberrant Plasma Cells; Anemia; Peripheral Blood Smear; Plasma Cell Leukemia
Abbreviations: PCL: Plasma Cell Leukemia, MG: Monoclonal Gammopathies, IMWG: International Myeloma Working Group, pPCL: Primary Plasma Cell Leukemia, MM: Multiple Myeloma, FISH: Fluorescence in Situ Hybridization
Introduction
The recent consensus recommendations from the International Myeloma Working Group (IMWG) regarding the diagnostic criteria for plasma cell leukemia (PCL) have sparked a debate about the diagnosis of this condition and the role of the percentage of circulating plasma cells in determining it. PCL is the most aggressive variant of monoclonal gammopathies (MG) and can either arise de novo (primary PCL, pPCL) or develop during advanced stages of multiple myeloma (MM) (secondary PCL) [1-10]. The importance of a prompt diagnosis of PCL lies in its significant impact on prognosis, making it crucial to establish the most effective treatment as soon as possible [1,3-5].
The original diagnostic criteria for PCL, established by Kyle in 1974 [1,4,11], required a circulating plasma cell percentage greater than 20% and an absolute count exceeding 2×109/L in peripheral blood [1-5,8]. However, in 2013, the IMWG cautioned that these criteria might be overly stringent and proposed that meeting either one of the criteria should be sufficient for a PCL diagnosis [1,3,4]. They did, however, emphasize the importance of prospective evaluation of this revised approach [1,3,5].
The latest report from the IMWG reviewed two series of MM patients to examine the correlation between the percentage of circulating plasma cells at diagnosis and the prognostic implications for these patients. The studies revealed that the presence of ≥ 5% circulating plasma cells had a comparable adverse prognostic impact to previously defined pPCL [2-5,10]. Consequently, the final recommendation from the IMWG is that patients with newly diagnosed MM who exhibit 5% or more plasma cells in peripheral blood should be classified as having pPCL [2].
Case Reports
We present three cases of pPCL in which the presence of > 5% plasma cells in peripheral blood was incidentally discovered during the investigation of anemia.
Patient 1
The first patient is a 69-year-old woman who had been experiencing chronic anemia for 7 months. The patient had a history of iron deficiency, treated with iron replacement therapy. However, the anemia persisted, leading to a referral to the hematology department.
Upon reviewing the patient’s medical records, it was noted that she had common variable immunodeficiency and was receiving intravenous human immunoglobulin treatment. During the physical examination, she reported experiencing worsening lower back pain. Laboratory analysis of the blood cell count revealed anemia (hemoglobin level of 98 mg/dl) with a normocytic normochromic pattern without any abnormalities in leukocyte parameters. Additionally, a peripheral blood smear showed the presence of 9% aberrant plasma cells with characteristics such as small to medium size, eccentric nucleus, and irregular cytoplasm (Figure 1).
Based on the findings from the peripheral blood smear, further investigations were conducted to assess the gammopathy (see Table 1). A bone marrow examination revealed a 70% infiltration of plasma cells with atypical morphology and a clonal aberrant immunophenotype (Table 1). Immunoelectrophoresis of both blood and urine samples did not indicate the presence of a monoclonal spike. Consequently, the final diagnosis was determined to be non-secretory pPCL.
After a 24-hour cell culture, a bone marrow karyotype analysis was conducted, and G-band staining was used for metaphase analysis. The results revealed the presence of three metaphases with a hyperdiploid clone. To further investigate the genetic abnormalities, targeted fluorescence in situ hybridization (FISH) was performed on plasma cells (selected using CD-138). The FISH analysis demonstrated a clonal gain and amplification of CKS1B in chromosome 1q21, consistent with the karyotype findings, which showed multiple copies of chromosome 1 (Table 1).
Patient 2
The second patient is a 69-year-old woman who was referred from outpatient care to the Hospital’s Hematology Department for a study of chronic anemia without responding to maturative factors or iron therapy. In terms of her medical history, she has a history of high blood pressure and osteoporosis. During the clinical examination, she exhibited mild asthenia but no other notable symptoms.
In the laboratory complete blood count, a normocytic, normochromic anemia of 100 mg/dL was observed, and no other significant parameters were found to be altered. Upon further examination of the blood smear, 5% of the cells were identified as plasma cells, and two erythroblasts were observed per 100 leukocytes. The plasma cells exhibited an atypical morphology, characterized by prominent cytoplasm and an eccentric nucleus (Figure 1). Based on these findings, a subsequent workup for MG was conducted, and the results are presented in Table 1.
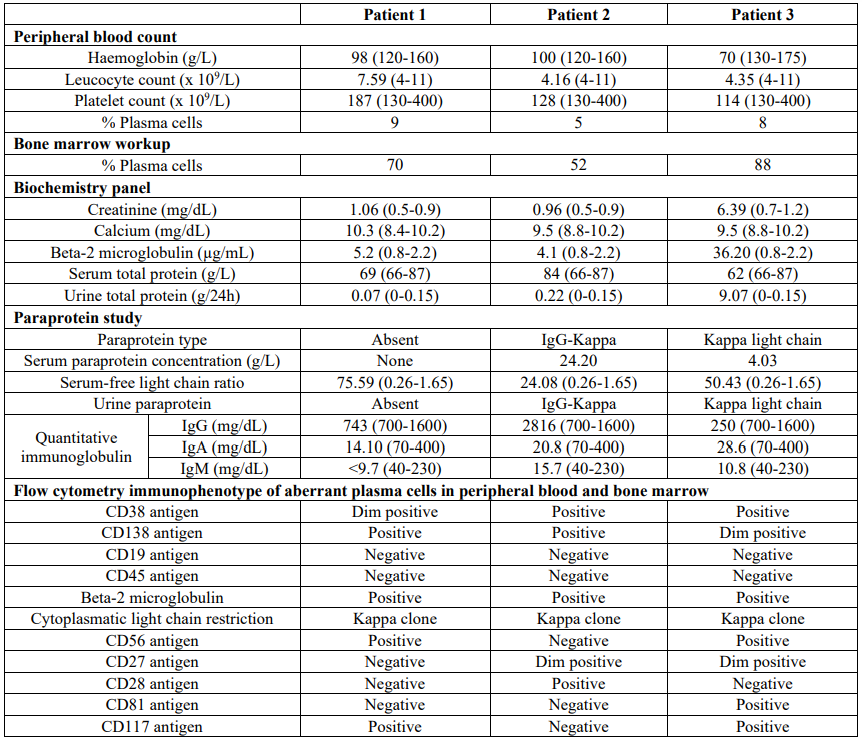
Table 1: Results of laboratory workup. Reference intervals are showed in parenthesis.
Serum protein electrophoresis confirmed the presence of an IgG kappa paraprotein, and the bone marrow study revealed infiltration of atypical plasma cells accounting for 52% of the sample. Further analysis through bone marrow and peripheral blood flow cytometry demonstrated the existence of a clonal population displaying an aberrant phenotype (Table 1). Additionally, bone marrow metaphase cytogenetics using G-band staining revealed a hyperdiploid karyotype with several structural chromosome abnormalities (Table 1).
Targeted plasma cell FISH analysis revealed a clonal gain and amplification in chromosome 1q21 (CKS1B) as well as the presence of a translocation (14;16)(q32;q23), indicated by the fusion of IGH/MAF genes.
Patient 3
The third patient was a 78-year-old man who was admitted to the emergency department due to dyspnea, peripheral edema, and significant weight loss over the past month. The patient’s medical history included high blood pressure, chronic anemia, hyperlipidemia, chronic obstructive pulmonary disease, nephrotic syndrome and chronic heart failure. The medical examination revealed worsening heart failure, along with acute renal failure (creatinine level of 6.64 mg/dL), hyperkalemia, and metabolic acidosis. Laboratory findings indicated a deterioration in the patient’s anemia, with a hemoglobin level of 77 g/L (previously 130 g/L) and a slight decrease in platelet count (114×109/L). A review of the peripheral blood smear revealed the presence of 8 aberrant plasma cells per 100 leukocytes. These plasma cells displayed atypical morphology, characterized by irregular cytoplasm and several with eccentric nuclei (Figure 1).
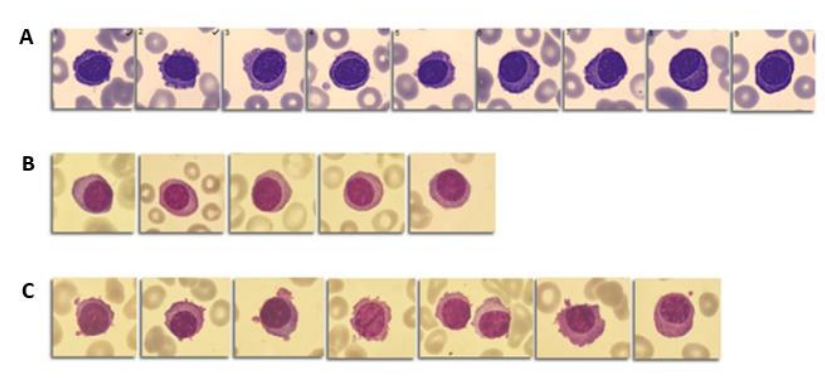
Figure 1: Aberrant plasma cells in peripheral blood smear. A: Patient 1 (9 %); B: Patient 2 (5 %); C: Patient 3 (8 %).
A comprehensive evaluation of MG was conducted, revealing the presence of a kappa light chain serum and urine paraprotein. An urgent bone marrow study yielded the following results: bone marrow infiltrated by 88% plasma cells with atypical morphology and a clonal immunophenotype (see Table 1). Metaphase karyotyping of the bone marrow exhibited a complex karyotype with several structural chromosome abnormalities. Targeted plasma cell FISH analysis indicated the presence of the following molecular abnormalities: deletion of chromosome 1p32 (CDKN2C), deletion in chromosome 17p13 (TP53), and t(11;14)(q13;q32). However, FISH analysis did not reveal t(14;16), but it did demonstrate a deletion in the MAF gene (16q23). The deletions of CDKN2C and MAF were consistent with the karyotype, showing a deletion in the short arm of chromosome 1 and a deletion in the long arm of chromosome 16 (Table 1).
Discussion
Based on the updated IMWG criteria, all the cases presented above should be classified as pPCL. pPCL is recognized as a separate clinicopathological entity from MM, characterized by distinct cytogenetic, molecular patterns, and clinical presentation. It represents one of the most difficult forms of MG to diagnose and manage due to its aggressive nature and poor response to conventional cytotoxic and novel therapeutic agents [1,2,4,10,12,13].
The diagnosis of PCL continues to rely on the standard morphological examination of peripheral blood [2,4,10]. Therefore, it is crucial for clinicians and specialists responsible for reviewing peripheral smears to recognize the significance of accurately identifying atypical plasma cells and understanding the clinical implications for the patient. Distinguishing atypical plasma cells from reactive plasma cells is important, as polyclonal plasma cells can be present in bacterial and viral infections, serum sickness, hyperimmunization, and certain autoimmune diseases [10,12]. To ensure that PCL diagnoses are not missed, guidelines recommend the routine review of peripheral blood smears for all patients with MM in clinical practice [2,4].
In all the cases presented, the detection of plasma cell dyscrasia resulted from examining the blood smear while investigating unclassified chronic anemia. This finding prompted the specialist to inform the clinician, initiating the workup for MG.
These cases underscore the significance of reviewing peripheral blood smears. Cytological examination of blood cells is an integral part of routine hematological investigations and offers valuable insights into cell morphology that cannot be captured by hematological analyzers alone. This aids in distinguishing and diagnosing various hematological disorders.
Flow cytometric immunophenotyping of peripheral and bone marrow blood was performed to identify and confirm the clonality of plasma cells. The plasma cells exhibited a strong expression of CD38 and CD138. Notably, all three patients demonstrated an aberrant loss of CD19 antigen and lacked CD45 expression. While it is generally reported that PCL exhibits a lower expression of CD56, indicative of a less mature plasma cell immunophenotype [1], our findings diverged in terms of CD56 expression, which aligns with observations from other studies [6]. Specifically, the plasma cells in the second patient lacked CD56 expression, displaying a complete pPCL immunophenotype consistent with previous reports (CD38+, CD138+, CD117-, CD56-, CD28+, CD27+) [1,8]. Additionally, the absence of CD56 is a common feature in myeloma with t(14;16) [1]. Conversely, the first and third patients exhibited an aberrant immunophenotype more characteristic of clonal plasma cells in MM [1,8].
The three cases described exhibited high-risk cytogenetics with karyotype and molecular abnormalities detected by FISH. The molecular characterization of pPCL can be further refined by the presence of specific translocations, with the most common being t(11;14) [1,3,5,7,8,13], which was only observed in our third patient. Adverse outcomes have been associated with t(14;16), which dysregulates MAF and was present in our second patient [7,9,10]. Additional molecular features linked to poor prognosis include aberrations in chromosome 1 (CKS1B amplifications and CDKN2C deletion), considered adverse cytogenetic abnormalities in MM and also frequently found in pPCL [4,8,13]. Patient three exhibited del(17p13) involving TP53, which is another poor prognostic molecular abnormality [4,8-10,13]. The term “double-hit” and “triple-hit” has emerged to describe these molecular abnormalities and has been shown to predict poor overall survival. However, the impact on survival from chromosome 1q21/CKS1B gain may vary depending on the underlying karyotype and the presence of other molecular aberrations, such as TP53 deletion [14].
Conclusion
We report three cases of pPCL diagnosed in our laboratory within a span of four months. A thorough examination of peripheral blood smears should be conducted in all patients suspected of having MG or even in those without any clinical suspicion but with unexplained anemia, as there is a possibility of underdiagnosing this entity with the new IMWG definition.
References
- Fernández de Larrea C, Kyle RA, Durie BGM, Ludwig H, Usmani S, et al. (2013) Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 27(4): 780-791. [PubMed.]
- Nandakumar B, Kumar SK, Dispenzieri A, Buadi FK, Dingli D, et al. (2021) Clinical characteristics and outcomes of patients with primary plasma cell leukemia in the era of novel agent therapy. Mayo Clin Proc. 96(3): 677-687. [PubMed.]
- Noel P, Kyle RA. (1987) Plasma cell leukemia: An evaluation of response to therapy. Am J Med. 83(6): 1062-1068. [PubMed.]
- Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, et al. (2018) Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 8(12): 116. [PubMed.]
- Kraj M, Kopeć-Szlęzak J, Pogłód R, Kruk B. (2011) Flow cytometric immunophenotypic characteristics of 36 cases of plasma cell leukemia. Leuk Res. 35(2): 169-176. [PubMed.]
- Gundesen MT, Lund T, Moeller HEH, Abildgaard N. (2019) Plasma cell leukemia: Definition, presentation, and treatment. Curr Oncol Rep. 21(1): 8. [Ref.]
- Tuazon SA, Holmberg LA, Nadeem O, Richardson PG. (2021) A clinical perspective on plasma cell leukemia) current status and future directions. Blood Cancer. 11(2): 23. [PubMed.]
- Chaulagain CP, Diacovo M-J, Van A, Martinez F, Fu C-L, et al. (2021) Management of primary plasma cell leukemia remains challenging even in the era of novel agents. Clin Med Insights Blood Disord. 14: 263485352199938. [PubMed.]
- Evans LA, Jevremovic D, Nandakumar B, Dispenzieri A, Buadi FK, et al. (2020) Utilizing multiparametric flow cytometry in the diagnosis of patients with primary plasma cell leukemia. Am J Hematol. 95(6): 637-642. [PubMed.]
- Nabeel H, Ahmed T, Senzel L. (2021) Pathologist review of peripheral blood smears containing plasma cells and plasmacytoid lymphocytes. Int J Lab Hematol. 43(2): 76-79. [PubMed.]
- Granell M, Calvo X, Garcia-Guiñón A, Escoda L, Abella E, et al. (2017) Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica. 102(6): 1099-1104. [PubMed.]
- Castaneda O, Baz R. (2019) Multiple Myeloma Genomics - A Concise Review. Acta Med Acad. 48(1): 57-67. [PubMed.]
- Mikulasova A, Morgan GJ, Walker BA. (2022) Chromosomal abnormalities in multiple myeloma. Nat Rev Dis Primers. 8(1): 42. [PubMed.]
- Gowin K, Skerget S, Keats JJ, Mikhael J, Cowan AJ. (2021) Plasma cell leukemia: A review of the molecular classification, diagnosis, and evidenced-based treatment. Leuk Res. 111(106687): 106687. [PubMed.]