>Corresponding Author : Paul Audu
>Article Type : Case Report
>Volume : 3 | Issue : 3
>Received Date : 28 March, 2023
>Accepted Date : 08 April, 2023
>Published Date : 13 April, 2023
>DOI : https://doi.org/10.54289/JCRMH2300115
>Citation : Audu P, Bolshem B, Audu G and Jankowitz B. Intranasal Administration of Local Anesthetic for Management of Post-Craniotomy Headache. J Case Rep Med Hist 3(3): doi https://doi.org/10.54289/JCRMH2300115
>Copyright : © 2023 Audu P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Department of Anesthesiology, Cooper University Hospital, I Cooper Plaza, Camden NJ 08103
2Department of Department of Anesthesiology, Long Island Jewish Medical Center, 270-05 76th Ave., New Hyde Park, NY, 11040
3Department of Cell Biology and Neuroscience, Rowan-Virtua School of Osteopathic Medicine, 1 Medical Center Dr, Stratford NJ 08084
4Department of Neurosurgery, Perelman School of Medicine at the University of Pennsylvania, 3400 Civic Center Boulevard, Philadelphia, PA 19104
*Corresponding author: Paul Audu, Department of Anesthesiology, Cooper University Hospital, I Cooper Plaza, Camden NJ 08103
Abbreviations: SPG: Sphenopalatine Ganglion, TG: Trigeminal, INALA: Intranasal Administration of Local Anesthetic, SPGB: Sphenopalatine Ganglion Block
Introduction
We present a case of a post craniotomy headache that was successfully treated with an intranasal sphenopalatine ganglion (SPG) block. The currently accepted mechanism of action of the SPG block does not explain its efficacy in the post-craniotomy setting. We suggest an alternate mechanism involving blockade of trigeminal (TG) neurotransmission and advocate its application in headache syndromes modulated by V1 and V2 branches of the TG nerve.
Case Report
A 50-year-old man with a 40 pack-year smoking history presented with a 5mm Right middle cerebral and 2mm anterior communicating artery aneurysm, discovered incidentally, during a workup for impotence. He was on Suboxone® for opiate use disorder. He underwent and uneventful pterional craniotomy and aneurysm clipping and was discharged home the following day. On his follow up visit on the ninth post-operative day, he complained of a severe (10/10) persistent right-sided headache that had remained unabated since his surgery. He described it as “internal”, “pounding” and distinct from incisional pain. A head CT revealed a Right temporo-parietal extra-axial fluid collection (Fig 1). It did not respond to over-the-counter analgesics. He was started as an outpatient on oxycodone which he was reluctant to take for fear of relapsing into opiate dependence. He presented to the emergency room a week later, unable to tolerate the pain. It was now associated with nausea, dizziness, and “seeing stars”. The extra-axial collection appeared unchanged by CT scan. He was offered the option of surgical evacuation of the subdural collection but he declined. He was started on Hydrocortisone, 1 mg/kg, using dosing guidelines of the SUCRE trial for chronic subdural hematomas [1]. He discontinued the treatment because he felt it was ineffective. On the 27th post-operative day, he was re-admitted with an unrelenting headache requiring parenteral opiates. He again refused surgery and indicated he did not want to take long-term opiates. As a last resort, he received a trial of intranasal administration of local anesthetic (INALA). A total of two ml of 4% lidocaine was instilled into either nares using a hollow shaft Q-tip [2]. The patient experienced transient dental anesthesia over his maxillary teeth but no immediate relief of his headache. However, the following morning, his headache had completely dissipated and he was discharged home. Two months after the block, he remains symptom free enjoying his prior quality of life.
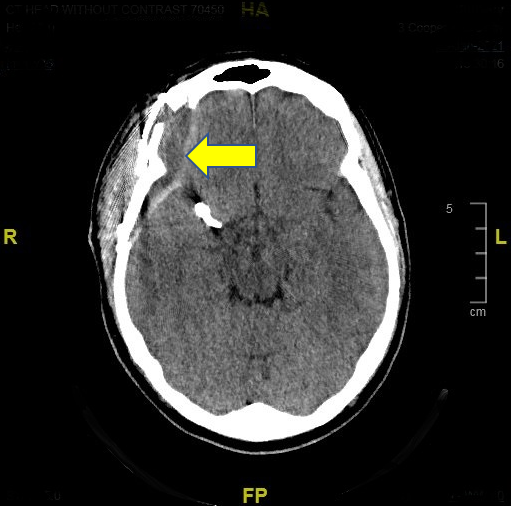
Figure 1: CT Head without Contrast; Arrow points to Sub-Dural Collection
Discussion
Moderate to severe post-operative pain is common following intracranial surgery [3-5] especially in the first 24 hours but it typically subsides by 72 hours. The patient’s post-operative pain persisted beyond what is typical. By his admission, its character transitioned from a somatic type, emanating from the scalp and soft-tissue, to a deeper seated, poorly localized “visceral” internal discomfort. We hypothesized that interrupting transmission via the trigeminal nerve might provide some relief. We performed INALA, not certain of its efficacy or the longevity of any relief. We were pleased with the results.
Local anesthetics have been applied intranasally for over a century under the guise of a “sphenopalatine ganglion block” (SPGB) [6]. The intervention has been used effectively in the treatment of migraines, cluster headaches, vascular headaches and post-dural puncture headaches [7]. The local anesthetic was hypothesized to trickle from the nasal cavity, through the sphenopalatine foramen and into the pterygopalatine fossa where the SPG resides. Blocking this major parasympathetic ganglion was thought to modulate vascular tone of the intracranial vessels and perhaps inhibit release of neurotransmitters involved in cerebrovascular nociception. This hypothesis has never been validated and has only recently been questioned [8]. The flawed paradigm has discouraged the application of this simple technique to a variety of pain syndromes for which INALA might be effective.
Closer scrutiny of animal data suggests that the efficacy of INALA might be related to blockade of the trigeminal nerve. The transfer of biologics from the nasal mucosa to the brain has been well documented [9-11]. Drugs travel by two pathways- the olfactory and trigeminal- bypassing the blood-brain barrier, reaching sites as distant as the forebrain, brain stem and rostral spinal cord within 30 minutes. In one animal study, investigators applied lidocaine to the nasal mucosa of rats and measured drug tissue concentrations at different sites [12]. Following IN application, the mean lidocaine concentration in the trigeminal ganglion was 147 µM compared with only 11 µM after intravenous administration of the same dose. This concentration of lidocaine within the trigeminal ganglion following intranasal injection falls within the range of concentrations necessary to block pain transmission along A-delta and C fibers (100-1400uM) [13] (Huang JH). Intravenous injection of lidocaine does not produce the same concentration within the trigeminal ganglion and thus, would not produce the same effect. Lidocaine concentration in the maxillary division of the TG nerve was 803 µM. This may explain the success of INALA in providing dental analgesia for maxillary teeth [14].
Extrapolating this animal data to humans, it is reasonable to surmise that INALA blocks nerve transmission in A-delta and unmyelinated C nociceptive nerve fibers in the trigeminal nerve and ganglion. Since the trigeminal nerve is the principal afferent pathway for transmission of visceral nociceptive afferents from the cranium [15], blocking this pathway may be the mode of analgesia in a variety of headache syndromes.
This would explain the efficacy of INALA in providing analgesia in our patient. The duration of analgesia was unexpected. Animal models suggest that nerve injury may result in excitability of ectopic neurons whose spontaneous firing amplify and sustain nociception even after resolution of the inciting injury [16]. In cats, chemical stimulation of dural receptive fields with inflammatory mediators sensitizes the trigeminal primary afferent neurons that innervate the meninges. Subsequent mechanical stimuli that previously elicited little or no response now result in an exaggerated response [17]. This phenomenon of central sensitization can be blocked by low concentrations of lidocaine [18-19], bupivacaine and tetrodotoxin [16]. In rats, tetrodotoxin suppressed spontaneous afferent neuronal activity for up to 27 days and prevented the development of neuropathic pain. These animal data may explain the prolonged analgesia derived from a single dose of local anesthetic in our patient.
Conclusion
INALA is a simple and safe technique that should be considered for patients with persistent headaches following intracranial surgery. Transient dental analgesia of maxillary teeth might be an indicator of successful block. Further studies are warranted to determine its utility in post-craniotomy pain syndromes.
Conflicts of Interest: The authors declare no conflicts of interest.
Funding: The authors have no source of funding to declare.
References
- Henaux P-L, Le Reste P-J, Laviolle B, et al. (2017) Steroids in chronic subdural hematomas (SUCRE trial): study protocol for a randomized controlled trial. Clinical Trial Trials. 18(1): 252. [Ref.]
- Windsor RE, Jahnke SS. (2004) Sphenopalatine Ganglion Blockade: A Review and Proposed Modification of the Transnasal Technique. Pain Physician. 7(2): 283-286. [PubMed.]
- Rocha-Filho PAS. (2015) Post-Crani Headache; Clinical view with a focus on persistence. The Journal of Head and Face Pain. 55(5): 733-738. [PubMed.]
- Lutman B, Bloom J, Nussenblatt B, et al. (2018) A Contemporary Perspective on the Management of Post-Craniotomy Headache and Pain. Curr Pain Headache Rep. 22(10): 69. [PubMed.]
- Magalhaes JE, Azevedo-Filho H, Rocha-Filho PAS. (2013) The risk of headache attributed to surgical treatment of Intracranial Aneurysms: A Cohort Study. Headache. 53(10): 1613-1623. [PubMed.]
- Sluder G. (1908) The role of the Sphenopalatine Ganglion in Nasal Headache. NY State J Med. 27: 8-13. [Ref.]
- Day M. (1999) Spenopalatine Ganglion Analgesia. Curr Rev Pain. 3(5): 342-347. [PubMed.]
- Narouze S. (2021) Topical intranasal lidocaine is not a sphenopalatine ganglion block. Regional Anesthesia & Pain Medicine. 46: 276-279. [PubMed.]
- Lochhead JL, Thorne RG. (2012) Intranasal delivery of biologics to the central nervous system. Advanced Drug delivery reviews. 64 (7): 614-628. [PubMed.]
- Thorne RG, Pronk GJ, et al. (2004) Delivery of Insulin-like Growth Factor-1 to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 127: 481-496. [PubMed.]
- Thorne RG, Hanson LR, Ross TM, et al. (2008) Delivery of Interferon to the monkey nervous system following intranasal administration. Neuroscience. 152: 785-797. [PubMed.]
- Johnson NJ, Hanson LR, and Frey WH. (2010) Trigeminal Pathways Deliver a Low Molecular Weight Drug from the Nose to the Brain and Orofacial Structures Molecular Pharmaceutics. 7 (3): 884-893. [Ref.]
- Huang JH, Thalhammer JG, et al. (1997) Susceptibility to Lidocaine of Impulses in Different Somatosensory Afferent Fibers of Rat Sciatic Nerve. Journal of Pharmacology and Experimental Therapeutics. 282(2): 802-811. [PubMed.]
- Hersh, Elliot V, et al. (2016) Intranasal tetracaine and oxymetazoline: a newly approved drug formulation that provides maxillary dental anesthesia without needles. Current medical research and opinion. 32(11): 1919-1925. [PubMed.]
- Messlinger K, Russo AFF. (2019) Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 39(13): 1661-1674. [PubMed.]
- Xie W, Strong JA, Meija JTA. (2006) Neuropathic pain: Early spontaneous afferent activity is the trigger Pain. 16(3): 243-256. [Ref.]
- Strassman AM, Raymond SA and Burstein R. (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 384 (12): 560-564. [PubMed.]
- Sotgiu MI, et al. (1992) Effect of Systemic Lidocaine on Dorsal Horn Neuron Hyperactivity Following Chronic Peripheral Nerve Injury in Rats. Somatosensory & Motor Research. 9(3): 227-233. [PubMed.]
- Kanai A, Hiruma H, Katakura T. Low-concentration Lidocaine Rapidly Inhibits Axonal Transport in Cultured Mouse Dorsal Root Ganglion Neurons. 95(3): 675-680. [PubMed.]