>Corresponding Author : Houssein Darwish
>Article Type : Case Report
>Volume : 3 | Issue : 2
>Received Date : 24 July, 2022
>Accepted Date : 07 Aug, 2022
>Published Date : 21 Feb, 2023
>DOI : https://doi.org/10.54289/JCRMH2300108
>Citation : Alawi M, Qronfla H, Begum A, Harraz MM, Elyamany M, et al. (2023) Association of Ultrasound Detected Axillary Lymphadenopathy and Anti-COVID-19 Vaccine Among Women Scanned in King Abdul Aziz Hospital, Makkah-A Pictorial Essay. J Case Rep Med Hist 3(2): doi https://doi.org/10.54289/JCRMH2300108
>Copyright : © 2023 Alawi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1KAAH (King Abdul-Aziz Hospital), Holy Makkah, Kingdom of Saudi Arabia
2Radiology Department, Mansoura University, Mansoura, Egypt
3King Abdul-Aziz University, Jeddah, Kingdom of Saudi Arabia
*Corresponding author: Malak Alawi, KAAH (King Abdul-Aziz Hospital), Holy Makkah, Kingdom of Saudi Arabia
Abbreviations: SBI: Society of Breast Imaging
Introduction
Unilateral axillary lymphadenopathy can pose to be diagnostic dilemma in breast ultrasound and may lead to unnecessary concern and biopsies.
Pfizer/BioNTech vaccine was approved for use in the UK on December 2, 2020 and Oxford Astra Zeneca vaccine was approved on December 30, 2020 [1]. There has been increase in the number of cases of unilateral axillary lymphadenopathy in patients post covid-19 vaccination [2,3].
The lymphadenopathy due to the vaccination is benign reactive hyperplasia due to its strong immune response and is resolved on follow up ultrasound with no need for biopsy or further workup [4]. Therefore, it is of utmost importance that all radiologists should be aware of such a possibility in order to reduce patient anxiety and unnecessary biopsies.
In this pictorial essay, we aim to highlight the various appearances of the unilateral axillary lymphadenopathy that were detected in patients with history of recent covid-19 vaccination that presented to our ultrasound clinic in KAAH hospital, Makkah during a 9 month period - from February till October,2021. In total, there were 18 patients. Most of the women, had symptoms of pain in the axilla and breast tenderness which started after the vaccination.
Ultrasound appearances
On ultrasound, the axillary lymph nodes showed mild enlargement in size, however the normal reniform shape was maintained and so was the fatty hilum. The cortical thickness was increased from 0.4 to 0.8cm on an average with mild increase in hilar vascularity (Fig1 and Fig2).
However, there was one patient who had completely rounded axillary lymphnodes with loss of fatty hilum and showing increased vascularity (Fig 3a and 3b).
Therefore, the ultrasound presentation of post COVID-19 vaccination axillary lymphadenopathy is mostly of that of benign reactive hyperplasia in which the shape of the lymphnode is maintained and fatty hilum is intact, and the cortex shows increased thickness. However, there are few cases in which the lymph nodes may assume a more sinister appearance of rounded shape, loss of hilum and increased cortical thickness and vascularity, similar to lymphnodal metastases.
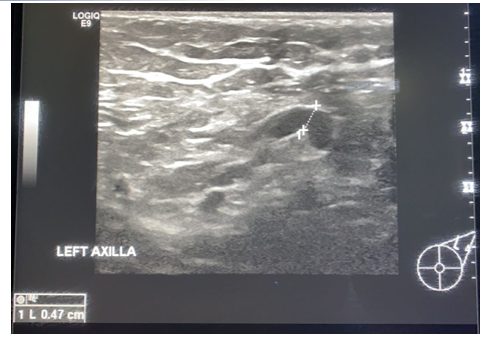
Figure 1. A 42 year old lady, came with complaint of left breast pain and lumpy feeling in the left breast, on ultrasound there was a left sided axillary lymphadenopathy with intact fatty hilum and maintained shape with increased cortical thickness-0.47cm-impressive of benign reactive hyperplasia.
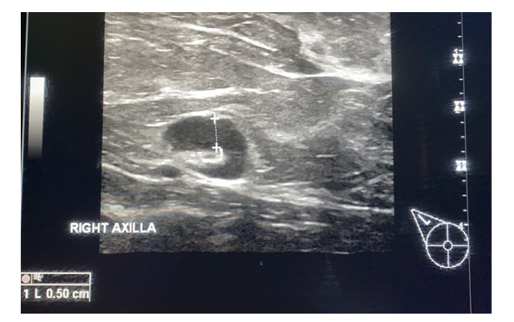
Figure 2. A 56 year old lady presented with right breast pain and recent history of COVID-19 vaccination to the right arm. Ultrasound showed enlarged axillary lymph node, maintained reniform shape, and increased cortical thickness-0.5cm, however the fatty hilum was intact-impressive benign reactive hyperplasia.
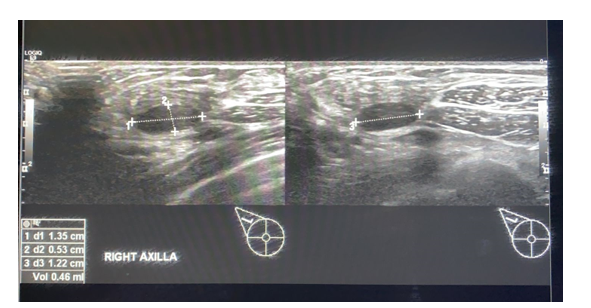
Figure 3a.
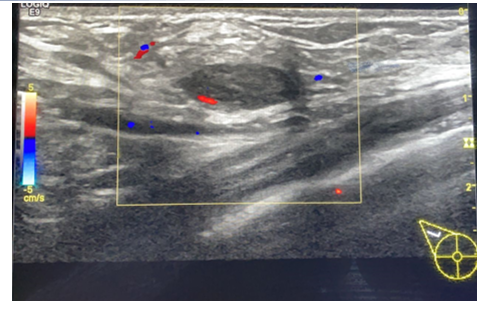
Figure 3b. A 26 year old lady with a recent history of COVID-19 vaccination, staff nurse in our hospital. The axillary lymph nodes showed loss of the normal reniform shape, increased cortical thickness with loss of fatty hilum and showing vascularity.
Therefore, the ultrasound presentation of post COVID-19 vaccination axillary lymphadenopathy is mostly of that of benign reactive hyperplasia in which the shape of the lymphnode is maintained and fatty hilum is intact, and the cortex shows increased thickness. However, there are few cases in which the lymph nodes may assume a more sinister appearance of rounded shape, loss of hilum and increased cortical thickness and vascularity, similar to lymphnodal metastases.
Discussion
Lymphadenopathy can result from a several causes ranging from benign causes such as infections, inflammations or traumatic injury to the chest wall, or arm or the breast, or autoimmune disease, to malignancy [5].
Unilateral axillary lymphadenopathy has been linked in the past to various vaccines, such as BCG vaccine, HPV vaccine [6].
And recently in the post COVID-19 vaccination era, after the approval of vaccination in Saudi Arabia [7].
Pfizer-BioNTech and Oxford AstraZeneca vaccines both have shown various side effects such as chills, fever, and unilateral axillary lymphadenopathy at the side of injection [8,9].
Ultrasound is the modality of choice for assessment of axillary lymph nodes due to its high soft - tissue resolution.
Axillary lymphadenopathy may pose as a diagnostic dilemma as the characteristics of the enlarged lymph nodes are non-specific and of varying sonographic appearance, ranging from benign reactive morphology to those of suspicious malignant appearance.
Sonographic appearances of COVID-19 vaccine-related lymphadenopathy are non-specific and include lymph node enlargement as measured by increased short-axis diameter, a thickened cortex and loss of the normal fatty hilum. The affected lymph node may be hypervascular on colour Doppler. The contralateral axillary lymph nodes should be of normal size in the absence of concurrent pathology.
A retrospective study was done for 34 patients to compare the different ultrasound parametric findings of axillary lymphadenopathy post COVID-19 vaccination. The study showed that after the first dose almost 70% patients developed axillary lymphadenopathy, while after the second dose, the prevalence was almost 46% and these patients presented with worrisome findings on ultrasound including the enlarged size, rounded shape, increased cortical thickness, distorted hilum etc. [10].
There have been many similar studies conducted in US and European countries showing the association of unilateral axillary lymphadenopathy and COVID-19 vaccination and the various imaging findings. However, no such study was found in the Middle East and particularly from Saudi Arabia [9,10]. Also, there is a need for studies with larger sample size.
The diagnostic challenge is to differentiate these cases from axillary lymph node metastases. According to the guidelines of the Society of Breast Imaging (SBI) published in January 2021, the women with unilateral axillary lymphadenopathy who have a history of receiving COVID-19 vaccination in the ipsilateral arm in the preceding 4 weeks should be followed up on ultrasound after 4-12 weeks of receiving the second dose of the vaccine.
Each patient’s information regarding the dose of vaccine and the date and laterality is to be obtained before the ultrasound exam. They advised to conduct the screening imaging before the first dose of vaccine is taken or atleast 4-6 weeks after the second dose [11].
A recent proposition was made in March 2021, by the Journal of the American College of Radiology, of using “BIRADS 2 Bengin “assessment with clinical follow up for isolated unilateral adenopathy after recent COVID-19 vaccination in the ipsilateral arm [12].
These guidelines would help to reduce the number of follow up examinations.
The guidelines for breast ultrasound screening and management strategies for axillary lymphadenopathy cases in post COVID-19 have been set based on the Western population. There is a dire need to set similar guidelines based on the Middle Eastern and Saudi population in order for the prevention of misdiagnosis of the lymphadenopathy as cancer metastases and avoid undue patient anxiety and unnecessary biopsies.
Acknowledgements: Not applicable
Funding: This study had no funding from any resource.
Contributions: MMH carried out the radiological studies, participated in design of the study, AB and HQ collected the patients’ data, MMH performed the statistical analysis. MA participated in the sequence alignment and drafted the manuscript. MH participated in the acquisition of data. HQ participated in the sequence alignment. ME participated in the design of the study and performed the statistical analysis. RB conceived of the study, participated in its design and coordination, and helped to draft the manuscript. AB wrote the paper with revision. All authors read and approved the final manuscript.
Ethics declarations
Ethics approval and consent to participate: This study was approved by the Research Ethics Committee of KAAH (King Abdul-Aziz Hospital) in Holy Makkah, Saudi Arabia.
Consent for publication: All patients written informed consent.
Competing interests: The authors declare that they have no competing interests.
References
- Medicines and Healthcare products Regulatory Agency. (2021) Regulatory approval of Pfizer/BioNTech vaccine for covid-19. GOV.UK. [Ref.]
- Washington T, Bryan R, Clemow C. (2021) Author Affiliations from the Department of Radiology. Adenopathy following covid-19 vaccination. Radiology. 299(3). [Ref.]
- Ozütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, et al. (2021) Lymphadenopathy in COVID-19 vaccine recipients: Diagnostic Dilemma in oncologic patients. Radiology. 300(1). [Ref.]
- Edmonds CE, Zuckerman SP, Conant EF. (2021) Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of COVID-19 vaccination. American Journal of Roentgenology. 217(4). [Ref.]
- Maini R, Nagalli S. (2021) Lymphadenopathy. National Center for Biotechnology Information. U.S. National Library of Medicine. [PubMed.]
- Faermann R, Nissan N, Halshtok-Neiman O, Shalmon A, Gotlieb M, Yagil Y, et al. (2021) Covid-19 vaccination induced lymphadenopathy in a specialized breast imaging clinic in Israel: Analysis of 163 cases. Academic radiology. U.S. National Library of Medicine. 28(9): 1191-1197. [PubMed.]
- Ministry Of Health Saudi Arabia. (2020) MOH starts registering citizens and residents for covid-19 vaccination. [Ref.]
- Centre for Disease Control and Prevention. (2022) Possible side effects after getting a COVID-19 vaccine. Centers for Disease Control and Prevention. [Ref.]
- Locklin JN, Woodard GA. (2021) Mammographic and sonographic findings in the breast and axillary tail following a COVID-19 vaccine. Clinical imaging. U.S. National Library of Medicine. 80: 202-204. [PubMed.]
- Cocco G, Pizzi AD, Fabiani S, Cocco N, Boccatonda A, et al. (2021) Lymphadenopathy After the Anti- Covid-19 Vaccine: Multiparametric Ultrasound Findings. Biology. U.S. National Library of Medicine. 10(7): 652. [PubMed.]
- Grimm L, Destounis S, Dogan B, et al. (2021) SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. Society of Breast Imaging Patient Care and Delivery Committee. [Ref.]
- Lehman CD, D'Alessandro HA, Mendoza DP, Succi MD, Kambadakone A, et al. (2021) Unilateral lymphadenopathy after COVID-19 vaccination: A practical management plan for radiologists across specialties. Journal of the American College of Radiology: JACR. U.S. National Library of Medicine. 18(6): 843-852. [PubMed.]