>Corresponding Author : Francisco Javier Gómez Alfonso
>Article Type : Case Report
>Volume : 3 | Issue : 2
>Received Date : 17 Jan, 2023
>Accepted Date : 31 Jan, 2023
>Published Date : 06 Feb, 2023
>DOI : https://doi.org/10.54289/JCRMH2300106
>Citation : Gomez FJ, Gonzalez P, Val FD, Lomas A, Lopez M, et al. (2023) Gastric Neuroendocrine Tumor Type 1: A Case Report. J Case Rep Med Hist 3(2): doi https://doi.org/10.54289/JCRMH2300106
>Copyright : © 2023 Gomez FJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case Report | Open Access | Full Text
1Endocrinology Service Hospital General La Mancha Centro. Alcazar de San Juan (Ciudad Real). Spain
2Health Center of San Clemente. San Clemente (Cuenca). Spain
*Corresponding author: Gomez FJ, Endocrinology Service Hospital General La Mancha Centro. Alcazar de San Juan (Ciudad Real). Spain
Abstract
33-year-old patient who is referred to Endocrinology Outpatient Consultation in March 2021 due to the finding in gastric polyp biopsy 9 mm sessile located in greater curvature, of low-grade neuroendocrine tumor (ki-67 < 3%) without infiltration of the muscularis layer own. It is followed jointly with the Digestive Service, performing mucosectomy in April 2021 on carcinoid in major curvature of the stomach, where there is no evidence of residual tumor in the anatomo-pathological study (A-P). After completing the study with other complementary tests, gastric neuroendocrine tumor type 1 is diagnosed against the background of chronic atrophic gastritis. At present, the patient continues with periodic gastroscopic check-ups scheduled by the Digestive Service.
Abbreviations: A-P: Anatomo-Pathological, NCCN: National Comprehensive Cancer Network, TGNE: Tumors Gastric Neuroendocrine, SSAS: Somatostatin Analogues
Introduction and Evolution
33-year-old patient who is referred to Endocrinology Outpatient Consultation in March 2021 due to the finding in gastric polyp biopsy 9 mm sessile located in greater curvature, low-grade neuroendocrine tumor (ki-67 < 3%) without infiltration of the muscularis layer propria. The patient has no personal history of interest, except for asymptomatic cholelithiasis, and was being studied in Digestive for dyspepsia and constipation. No evidence of digestive bleeding or peptic ulcers; He does report ardors with some frequency. Actually, she was not being treated with antacids or prokinetics. The patient was very distressed by the possibility of surgery.
It provides an Octreoscan/SPECT-CT scan performed on March 21 with uptake at the level of the gastric antrum without other findings. Initially, and following the recommendations of the NCCN (National Comprehensive Cancer Network) guideline, followup endoscopic was proposed and mucosectomy was performed in April 2021 in major gastric curvature on carcinoid tumor, where there is no evidence residual tumor in the histological study, chronic gastritis with intestinal metaplasia is observed.
In the analytics of April 2021 highlights hypergastrinemia with elevated chromogranin A and low levels of vitamin B12, with iPTH and complete pituitary profile normal (see below). It is decided to start treatment with parenteral vitamin B12 and it postpone therapy with somatostatin analogues depending on of clinical evolution, also taking into account the existence of lithiasis in the gallbladder. In September 2021, a new endoscopy is performed, where correct scarring from previous mucosectomy is observed and sessile polyp of 2-3 mm is removed in gastric fundus. The A-P gastric biopsy study (antrum, fundus and mucosectomy scar) is reported as free of carcinoid tumor, with signs of chronic gastritis and H. pylori negative. In January 2022, the patient remains asymptomatic, provides thoraco-abdominal CT where it is not appreciated visceral lesions or suspicious lymphadenopathy, and Octreoscan/SPECT-CT where two small deposits with discrete increase in activity in body and gastric antrum, of doubtful significance for the patient's basic process, without others significant pathological findings.
Exploration And Complementary Tests
Laboratory test (April 2021): GFR >90 ml/min, corrected Ca 9.9 mg/dL , P 3.8, mg/dL, B12 100 pg/mL, gastrin 896 pg/mL, CgA 355 ng/mL, normal complete pituitary profile, iPTH 34 pg/mL, 5-hydroxyindoleacetic acid 1.9 mg/24 h (N < 6), positive intrinsic factor.
Laboratory test (January 2022): Normal blood count, glu 86mg/dL, GFR > 90 ml/min, LDLc 48 mg/dL, Tg 100 mg/dL, prot T 6.9 g/dL, HbA1c 4.6%, gastrin 998 pg/mL, CgA 232 ng/mL , vit B12 135 pg/mL, TSH 2.66 uIU/mL, vit D 22 ng/dL.
Gastroscopy (January 2021) (image 1): Esophagus: normal mucosa and caliber, cardia at the hiatal level. Stomach: mucosa of fundus, body and antrum normal. In body, curvature major, is removed with diathermy loop, after elevation with Orise, a sesil polyp of about 9 mm, then hemoclip is placed and recovers (B4). Adequate adherence of the cardia to the endoscope in retrovision. Patent pylorus. Duodenum: bulb and up to 2nd portion normal.
Gastroscopy (April 2021): Esophagus: normal. Stomach: normal fundus. Mucosectomy is performed by Duette on carcinoid of proximal gastric body, anterior face major curvature, administering argon at the base and subsequently applying Hemospray.
Gastroscopy (September 2021): Esophagus: normal. Stomach: in fundus, a sesile polyp of 2-3 mm. of carcinoid appearance, which is biopsied and removed, shining the base with argon and placing hemoclips. Correct scar of the 2nd mucosectomy that was performed, which is biopsied. Rest of normal body and antrum. Normal pylorus.
A-P Study (March 2021): BIOPSY GASTRIC MAJOR CURVATURE: WELLDIFFERENTIATED GASTRIC NEUROENDOCRINE NEOPLASIA WITH: Histological grade G1: < 2 mitosis /2mm . Proliferative index,Ki67: < 3 %. It expresses neuroendocrine markers: Chromogranin+/Synaptophysin+/CD56+. Extensive infiltration of the referred sample including muscularis mucosae and focally submucosal (pT1) The sample referred does not includes muscularis layer propria (assessment of limited extent by incisional biopsy).
A-P Study (April 21): GASTRIC BIOPSY (MUCOSECTOMY): - No residual neoplastic lesion is identified. - Antral gastric mucosa and submucosa with slight inflammatory activity chronic and focal intestinal metaplasia. - H. pylori are not identified. - Focal flare device.
A-P Study (September 21): A) GASTRIC BIOPSY (ANTRUM): - Chronic antral gastritis with foci of intestinal metaplasia. - Negative for H. pylori. - No evidence of carcinoid tumor. B) GASTRIC BIOPSY (FUNDUS): - Chronic atrophic gastritis. Negative for H. pylori. - No evidence of tumor carcinoid. C) GASTRIC BIOPSY (SCAR FROM PREVIOUS MUCOSECTOMY): - Fragments of gastric mucosa with slight reaction scarring fibroblast of the lamina propria with no evidence of residual carcinoid tumor.
Thoraco-abdominal CT scan (December 2021): Axillary and mediastinal chains without suspicious adenopathies or nodules. Clear lung parenchyma without condensation or nodules suspects. Fluid-free pleural cavity. Gastric chamber without wall thickenings. Embolization clip in minor curvature. Homogeneous liver without focal lesions. There is no bile duct dilation. Pancreas, spleen and adrenals without alterations. Kidneys without excretory pathway dilation. Non-conspicuous internal genitalia. There are no abdominal lymphadenopathy or free fluid. Bone frame without alterations.
Conclusion: No suspicious visceral lesions or lymphadenopathy were observed. There are no inflammatory changes.
Octreoscan/SPECT-CT (March 21) (image 2): Planar images show a physiological distribution of the radiopharmaceutical without that radiotracer deposits suggestive of pathological lesions are identified. When performing SPECT-CT, focal area is displayed with increased radiotracer activity that appears to correspond to the gastric antrum. In the rest of the images obtained, not displayed other radiotracer deposits suggestive of lesions with somatostatin receptor overexpression.
Conclusion: Focal Area In Gastric Antrum With Overexpression Of Somatostatin Receptors. Without Other Findings.
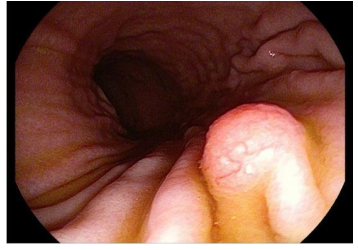
Image 1. Gastric polyp
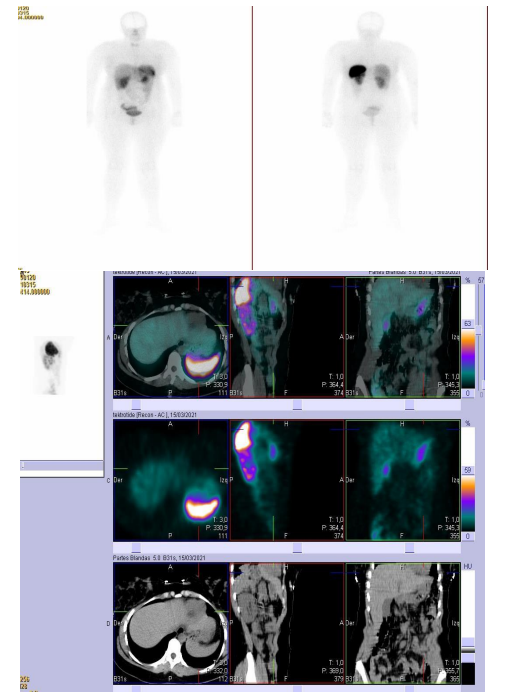
Image 2. Octreoscan/SPECT-CT (March 21)
Octreoscan/SPECT-CT (January 22) (image 3): In the planar images obtained, no findings are observed significant pathological. In the tomographic study, two small deposits with a discrete increase in activity were observed, with focal characteristics that seem to be located in the upper region of the anterior wall of the gastric body and the area of the antrum gastric, findings of dubious significance for the basic process. No other significant pathological findings
Conclusion: Findings Described In Body And Gastric Antrum, Of Dubious Significance For The Patient's Basic Process, To Be Assessed Through Directed Study And/Or Follow-Up.
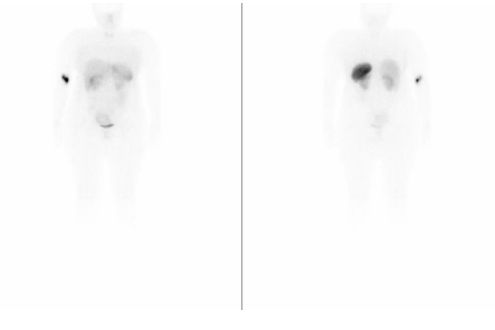
Image 3. Octreoscan/SPECT-CT (January 22)
Treatment
None
Discussion
Gastric neuroendocrine cells account for 1.2% of the entire volume of gastric epithelium; 30-35% are of the enterochromaffin type (ECL), which are located in the fundus and interact with other cells of the same system secreting histamine. Tumors gastric neuroendocrine (TGNE) are the most frequent gastroenteropathic neuroendocrine tumors (TGEPNE); They are usually benign and silent, but can also occur aggressively and clinically resemble adenocarcinoma. The TGNE can be divided into three types: type 1, associated with chronic autoimmune atrophic gastritis (type A), with chronic hypergastrinemia and achlorhydria, excellent prognosis and 100% 24-year survival; type 2, associated with Zollinger-Ellison syndrome (MEN-1), and type 3, which is usually sporadic with large high-grade lesions and metastatic potential [1-6]. TGNE type 1 accounts for 70-80% of TGNE [7-8], is more common in women, and is usually associated with other autoimmune diseases [9-10]. They are usually small (< 1-2 cm), multiple in 65%, and polypoid in 78% of cases. In relation to treatment, conservative therapy is preferred, since frequency of invasion of the muscle layer and metastasis is very low, as the case that represents us. For tumors < 1 cm, it prefers endoscopic resection, while in tumors > 1 cm or with extension of the muscle layer and / or invasion it is recommended surgical resection or antrectomy, the latter to avoid chronic stimulation of ECL cells. Ulceration of the lesion as well as a normal gastrin are considered a factor of poor prognosis suggestive of disseminated disease [11-12]. In our patient it was decided perform mucosectomy in greater gastric curvature for better staging, taking into account that the initial incisional biopsy on 9 mm sessile polyp did not include muscularis layer propria (see study A-P above). Somatostatin analogues (SSAS) may be helpful to treat multiple small lesions that are difficult to eradicate by endoscopy. In our case, in the absence of a tumor residual in mucosectomy specimen (April 21), as well as in sessile polyp biopsy (Sept 21), in addition to the presence of cholelithiasis that presented our patient, it was decided to defer this therapy with ASS according to clinical evolution. At the present time, it is asymptomatic and is followed jointly with the Digestive Service, who schedules periodic endoscopies every 3-6 months.
References
- Fave GD, O'Toole D, Sundin A, Taal B, Ferolla P, et al. (2016) ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 103: 119-124. [PubMed.]
- (2016) Guide practice of diagnosis, treatment and monitoring of neuroendocrine tumors. 3rd Edition. EDITA: Edika Med SL. [Ref.]
- García-Carbonero R, Vilardell F, Jiménez-Fonseca P, González-Campora R, González E, et al. (2014) Guidelines for biomarker testing in gastroenteropancreatic neuroendocrine neoplasms: a national consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 16: 243-256. [PubMed.]
- García-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Prado MPND, et al. (2010) Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 21: 1794-1803. [PubMed.]
- Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, et al. (2012) Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 61(1): 6-32. [Ref.]
- Piris MA, González E. (2016) Manual of help in the diagnosis pathological of TGEPNE. Ediciones Mayo, S.A. [Ref.]
- Borch K, Ahrén B, Ahlman H, et al. (2005) Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 242(1): 64-73. [PubMed.]
- Sculdo D, Bilgrami S. (1997) Pernicious anemia and gastric carcinoid tumor: case report and review. Am J Gastroenterol. 92(8): 1378-1380. [PubMed.]
- Rindi G, Bordi C, Rappel S, et al. (1996) Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 20(2): 168-172 [PubMed.]
- Thomas RM, Baybick JH, Elsayed AM, Sobin LH. (1994) Gastric carcinoids. An immunohistochemical and clinicopathologic study of 104 patients. Cancer. 73(8): 2053-2058. [PubMed.]
- Noh JH, Kim DH, Yoon H, et al. (2021) Clinical outcomes of endoscopic treatment for Type 1 Gastric Neuroendocrine. Tumor J. Gastrointest Surg. 25(10): 2495-2502. [PubMed.]
- Hanna A, Kim-Kiselac C, Tang R, et al. (2021) Gastric Neuroendocrine Tumors: Reappraisal of Type in Predicting Outcome. Ann Surg Oncol. 28(13): 8838-8846. [PubMed.]