>Corresponding Author : Gian Maria Pacifici
>Article Type : Mini Review
>Volume : 2 | Issue : 1
>Received Date : 24 March, 2022
>Accepted Date : 04 April, 2022
>Published Date : 07 April, 2022
>DOI : https://doi.org/10.54289/JCRMH2200103
>Citation : Pacifici GM. (2022) Clinical Pharmacology of Ampicillin. J Case Rep Med Hist 2(1): doi https://doi.org/10.54289/JCRMH2200103
>Copyright : © 2022 Pacifici GM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mini Review Article | Open Access | Full Text
Gian Maria Pacifici, via Sant’Andrea 32, 56127 Pisa, Italy
*Corresponding author: Gian Maria Pacifici, via Sant’Andrea 32, 56127 Pisa, Italy
Abstract
Ampicillin is active against gram-negative and gram-positive organisms. Ampicillin is a β-lactam antibiotic and β-lactam ring is destroyed by β-lactamases thus ampicillin is co-formulated with sulbactam an inhibitor of β-lactamases. Ampicillin has been found efficacy and safe in treatment of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis and ampicillin/sulbactam has been found efficacy and safe in treatment of multidrug-resistant Acinetobacter baumannii. Ampicillin treats sinusitis, otitis media, acute exacerbations of chronic bronchitis, epiglottitis, and bacterial meningitis and ampicillin/sulbactam treats upper respiratory-tract and skin infections, and many life-threatening infections. Ampicillin and sulbactam penetrates into body-tissues. Ampicillin and sulbactam elimination half-life is 1.41 and 1.73 hours, respectively, in healthy subjects, but the half-life of ampicillin and sulbactam is increased in patients with acute kidney injury undergoing dialysis. The prophylaxis with ampicillin/sulbactam prevents infections in patients undergoing surgery and the treatment with ampicillin or ampicillin/sulbactam has been studied. Ampicillin penetrates into cerebrospinal fluid in significant amounts and ampicillin treats the bacterial meningitis caused by Haemophilus influenzae type b and Haemophilus meningitis but ampicillin may become resistant to Streptococcus aureus, Klebsiella, Pseudomonas, Salmonella enterica serotype Typhimurium, and Shigella dysenteriae. Ampicillin freely crosses the human placenta and migrates into breast-milk is significant amounts. The aim of this study is to review ampicillin and ampicillin/sulbactam efficacy and safety, ampicillin and sulbactam penetration into body-tissues, ampicillin and sulbactam pharmacokinetics, ampicillin/sulbactam prophylaxis and treatment, treatment of bacterial meningitis with ampicillin, ampicillin transfer across the human placenta, ampicillin migration into the breast-milk, and ampicillin resistance.
Keywords: Ampicillin; breast-milk; cerebrospinal-fluid; efficacy-safety; meningitis; placenta; pharmacokinetics; prophylaxis; resistance; sulbactam; tissue-concentration; treatment
Abbreviations: CSF: Cerebrospinal Fluid
Introduction
Antimicrobial activity of ampicillin
Ampicillin is generally bactericidal for sensitive gam-positive and gram-negative bacteria. The meningococci and Listeria monocytogenes are sensitive. Many pneumococcal isolates have varying levels of resistance, and penicillin-resistant strains should be considered resistant. Haemophilus influenzae and the viridians group of streptococci exhibit varying degrees of resistance. Enterococci are about twice sensitive as they are to penicillin G. From 30 to 50% of Escherichia coli, a significant number of Pseudomonas mirabilis and practically all species of Klebsiella are resistant. Most strains of Shigella, Pseudomonas, Serratia, Acinetobacter, Bacillus fragilis, and indole-positive Proteus also are resistant. Resistant strains of Salmonella are recovered with increasing frequency. Concurrent administration of a β-lactamase inhibitor such as clavulanate or sulbactam markedly expands their spectrum of activity, particularly against Haemophilus influenzae, Escherichia coli, Proteus, and Bacillus fragilis [1].
Therapeutic indication of ampicillin
Ampicillin is active against Streptococcus pyogenes, many strains of Streptococcus pneumoniae and Haemophilus influenzae. Ampicillin constitutes effective therapy for sinusitis, otitis media, acute exacerbations of chronic bronchitis, and epiglottitis caused by sensitive organisms. Ampicillin-resistance Haemophilus influenzae is a problem in many areas and the addition of a β-lactamase inhibitor such as sulbactam extends the spectrum to β-lactamase-producing Haemophilus influenzae and Moraxella [1].
Treatment of urinary-tract infections
Most uncomplicated urinary-tract infections are caused by Enterobacteriaceae, Escherichia coli is the most common species. Ampicillin can be an effective agent for urinary-tract infections, but the high prevalence of resistance amongst Escherichia coli and Klebsiella makes the empiric use of ampicillin for urinary tract-infections challenging. Enterococcal urinary tract-infections are treated effectively with ampicillin alone [1].
Treatment of meningitis
Acute bacterial meningitis in children is a frequency due to Streptococcus pneumoniae or Neisseria meningitis. Because 20% to 30% of strains of Streptococcus pneumoniae now may be resistant to ampicillin, it is indicated for empiric single-agent treatment of meningitis. Ampicillin has excellent activity against Listeria monocytogenes, a cause of meningitis in immunocompromised patients. The combination of ampicillin and vancomycin plus a third-generation cephalosporin is a recommended regimen for empirical treatment of suspected bacterial meningitis in patients at risk for Listeria monocytogenes [1].
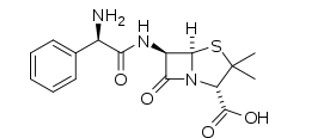
Ampicillin molecular structure (molecular weight = 349.406 grams/mole)
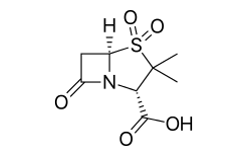
Sulbactam molecular structure (molecular weight = 233.243 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “ampicillin efficacy safety”, “ampicillin tissue concentration”, “ampicillin pharmacokinetics”, “ampicillin prophylaxis” “ampicillin treatment”, “ampicillin CSF”, “ampicillin meningitis”, “ampicillin resistance”, “ampicillin placental transfer”, and “ampicillin breast-milk”. In addition, the book “The pharmacological basis of therapeutics” [1] has been consulted.
Results
Ampicillin and ampicillin/sulbactam efficacy and safety
Ampicillin is effective and safe for treating 92.3% subjects infected by Streptococcus pneumoniae, 83.3% subject infected by Haemophilus influenzae, and 87.5% subjects infected by Moraxella catarrhalis [2]. High-dose ampicillin/sulbactam is a safe and effective treatment of critically ill patients with multidrug-resistant Acinetobacter baumannii [3]. Ampicillin/sulbactam is safe and effective as amoxicillin/clavulanate in the empiric treatment of upper respiratory-tract infections in adults [4]. Both ampicillin/sulbactam and cefuroxime provide safe and effective parenteral antibiotic therapy in paediatric patients with serious skin and skin structure infections [5]. Ampicillin/sulbactam is effective and safe agent in management of many life-threatening infections in paediatric patients [6]. Ampicillin/sulbactam is an efficacious and safe treatment of infections in diabetic foot [7].
Ampicillin and sulbactam concentrations in tissues
Twenty-four patients were treated with 2 grams of ampicillin and 1 gram of sulbactam intravenously and the concentrations of ampicillin and sulbactam were measured in different tissues. About 1 hour after dosing, ampicillin and sulbactam concentrations are 59.2 and 31.6 µg/ml, respectively, in serum and 33.5 and 19.5 µg/g, respectively, in different tissues. Ampicillin and sulbactam penetrate into tissues and maintain a ratio of 2:1 [8]. Nineteen patients undergoing prostatectomy were treated with 2 grams of ampicillin and 1 gram of sulbactam intravenously. The prostate concentration of ampicillin ranges from 0.42 to 54 mg/gram (median, 47 mg/gram) and the prostate concentration of sulbactam ranges from 0.15 to 24 mg/gram (median, 19 mg/gram) [9]. Ampicillin was administered orally at a dose of 500 mg in presence or absence of khat chewing. The bioavailability of ampicillin is significantly reduced when it is taken with khat chewing [10]. Ampicillin was administered intravenously at a dose of 250 mg to patients with sinusitis and the mean concentration of ampicillin in the nasal polypus is 1.67 µg/g [11].Pharmacokinetics of ampicillin and sulbactam in patients with acute kidney injury submitted to dialysis and in health subjects
Lorenzen et al. [12] studied the pharmacokinetics of ampicillin in 12 patients undergoing extended dialysis, in 4 patients undergoing intermittent dialysis, and in 6 healthy subjects. A single oral dose of ampicillin (2 grams) and sulbactam (1 gram) was administered to patients and healthy subjects.
Table 1. Pharmacokinetic parameters of ampicillin which are obtained in 12 patients undergoing extended dialysis, in 4 patients undergoing intermittent dialysis, and in 6 health subjects. A single oral dose of amoxicillin (2 grams) and sulbactam (1 gram) was administered to patients and healthy subjects. Values are the mean ± SD or range, by Lorenzen et al. [12].
| Parameter | Extended dialysis patients (N = 12) | Intermittent dialysis patients (N = 4) | Healthy subjects (N = 6) |
|---|---|---|---|
| Peak concentration (µg/ml) | 281+175 | --- | 82.1 - 154 |
| Tmax(h) | 0.5 | 0.25 | 0.25 |
| AUC (µg*h/ml) | 847+499 | 1,655+1,170 | 185+30.1 |
| Elimination half-life (h) | 2.8+0.8 | 2.22+0.92a 17.39+7.94b | 1.41+0.65 |
| Distribution volume (L) | 13.1+11.1 | --- | --- |
| Distribution volume (L/kg) | 0.6+0.10 | 0.40+0.10 | 0.22+0.04 |
| Total body clearance (ml/min) | 61.1+7.7 | 31.0+21.0b | 219+52.4 |
| Dialyzer clearance (ml/min) | 80.1+7.7 | 51.4+2.4 | N/A |
Tmax = time to reach the peak concentration. aOn dialysis. bOff dialysis. N/A = not applicable.
This table shows that ampicillin peak concentration is higher in patients undergoing extended dialysis than in healthy subjects, the time to reach ampicillin peak concentration is longer in patients undergoing extended dialysis than in patients undergoing intermittent dialysis and in healthy subjects, ampicillin AUC is higher in patients undergoing extended dialysis and in patients undergoing intermittent dialysis than in healthy subjects, ampicillin elimination half-life is longer in patients undergoing extended dialysis and in patients undergoing intermittent dialysis than in healthy subjects, ampicillin distribution volume, expressed as L/kg, is higher in patients undergoing extended dialysis and in patients undergoing intermittent dialysis than in healthy subjects, ampicillin total body clearance is lower in patients undergoing extended dialysis and in patient undergoing intermittent dialysis than in healthy subjects. These differences are due to the dialysis procedures. In addition, there is a remarkable interindividual variability of ampicillin pharmacokinetic parameters in both patients undergoing extended dialysis and patients undergoing intermittent dialysis. This variability is accounted by the patient diseases and dialysis procedures.
Table 2. Pharmacokinetic parameters of sulbactam which are obtained in 12 patients undergoing extended dialysis, in 4 patients undergoing intermittent dialysis, and in 6 health subjects. A single oral dose of amoxicillin (2 grams) and sulbactam (1 gram) was administered to patients and healthy subjects. Values are the mean ± SD or range, by Lorenzen et al. [12].
| Parameter | Extended dialysis patients (N = 12) | Intermittent dialysis patients (N = 4) | Healthy subjects (N = 6) |
|---|---|---|---|
| Peak concentration (µg/ml) | 88.1+47.5 | --- | 32.0 - 93.7 |
| Tmax (h) | 0.5 | 0.25 | 0.25 |
| AUC (µg*h/ml) | 324+190 | 432+206 | 85.5+23.3 |
| Elimination half-life (h) | 3.5+1.5 | 2.27+0.64a 13.36+7.39b | 1.73+0.72 |
| Distribution volume (L) | 22.0+21.8 | --- | --- |
| Distribution volume (L/kg) | 0.27+0.23 | 0.59+0.20 | 0.30+0.12 |
| Total body clearance (ml/min) | 81.1+81.7 | 45.3+19.5b | 217+97.2 |
| Dialyzer clearance (ml/min) | 83+12.1 | 75.8+ 27.1 | N/A |
Tmax = time to reach the peak concentration. aOn dialysis. bOff dialysis. N/A = not applicable.
This table shows that sulbactam peak concentration is similar in patients undergoing extended dialysis and in healthy subjects, the time to reach sulbactam peak concentration is longer in patients undergoing extended dialysis than in patients undergoing intermittent dialysis and heath subjects, sulbactam AUC is higher in patients undergoing extended dialysis and in patients undergoing intermittent dialysis than in healthy subjects, sulbactam elimination half-life is longer in patients undergoing extended dialysis and in patients undergoing intermittent dialysis than in healthy subjects, sulbactam distribution volume, expressed as L/kg, is higher in patients undergoing intermittent dialysis than in patients undergoing extended dialysis and healthy subjects, sulbactam total body clearance is lower in patients undergoing extended dialysis and in patients undergoing intermittent dialysis than in healthy subjects. These differences are due to the dialysis procedures. In addition, there is a remarkable interindividual variability in sulbactam pharmacokinetic parameters in both patients undergoing extended dialysis and in patients undergoing intermittent dialysis. This variability is accounted by the patient diseases and the dialysis procedures.
Prophylaxis with ampicillin/sulbactam or ampicillin prevents infections occurring during surgery
Ampicillin (1 gram) and sulbactam (0.5 grams), given intravenously twice daily, prevent infections in patients undergoing cardiovascular surgery [13]. Prophylactic ampicillin/sulbactam is safe and effective as cefuroxime for the prevention of infections in women submitted to Caesarean delivery [14]. Single-dose ampicillin/sulbactam provides better prophylaxis than single-dose ampicillin in women undergoing Caesarean delivery [15]. Prophylaxis with single-dose ampicillin is more effective than standard penicillin prophylaxis in preventing infections during surgery [16]. Prophylaxis with cefotaxime or ampicillin/tinidazole is equally effective in preventing infections during hysterectomy [17]. Ampicillin, administered orally at a dose of 1 gram twice daily, prevents chronic bronchitis [18]. Prophylactic ampicillin, administered orally at a dose of 1 gram twice daily, prevents infections during gastric surgery [19].
Treatment of infections with ampicillin or ampicillin/sulbactam and comparison of treatment efficacy with other antibiotics
Clinical and bacteriological results support the use of high dose of ampicillin/sulbactam in the treatment of multidrug-resistant Acinetobacter baumannii [20]. Ampicillin/sulbactam treats aspiration-associated pulmonary infections [21]. Ampicillin-ceftriaxone is a synergistic combination to treat orthopaedic infections due to Enterococcus faecalis [22]. Ampicillin/sulbactam effectively treats urinary-tract, skin, soft tissue, bones and joints, respiratory-tract, ears, nose, intra-abdominal, obstetric, and gynaecological infections, and septicaemia [23]. Eight patients with urinary-tract infections were treated with 500 mg of ampicillin or with 250 mg of cefaclor. The overall success rate in the cefaclor group is 75.7% and in the ampicillin, group is 79.4%. Thus, ampicillin is effective as cefaclor for treating urinary-tract infections [24]. Fifteen children were treated with ampicillin and 15 children received amoxicillin and both antibiotics were administered at a dose of 100 mg/kg daily for 5 days. Ampicillin is effective as amoxicillin in treating infections caused by Salmonella gastroenteritis [25]. Twenty-eight children, infected by Haemophilus influenzae type b meningitis, were treated with ampicillin at a dose of 100 mg/kg for 5 days or with rifampin at a dose of 20 mg/kg for 4 days. Rifampin may be more effective than ampicillin in treatment of Haemophilus influenzae type b meningitis [26].
Penetration of ampicillin into the cerebrospinal fluid (CSF)
Ampicillin tends to produce higher CSF concentrations than amoxicillin whereas the blood concentrations of amoxicillin and ampicillin are equal [27]. In 5 patients with listeric meningitis, the CSF concentration of ampicillin correlates with serum concentrations. About 40 min after intravenous administration of ampicillin, given at a dose of 50 to 60 mg/kg, the CSF concentration of ampicillin ranged between 5 and 8 µg/ml, which means a CSF to serum concentration ration of 3% to 5%. In a patient with renal failure, the CSF concentration of ampicillin ranged between 60 and 130 µg/ml indicating a CSF to serum concentration ratio of 40% to 87%. Ampicillin successfully treats listeric meningitis in all occasions [28]. Thirty-five newborns with septicaemia, caused by Staphylococcus aureus, were randomized to receive tobramycin, or ceftazidime or ampicillin. The CSF concentration of tobramycin were below 0.5 µg/ml, the CSF concentration of ceftazidime ranged between 2.5 to 17 µg/ml. The CSF concentration of ampicillin ranged from 1 to 80 µg/ml and ampicillin treats the meningitis caused by Streptococcus aureus, enterococci and Listeria meningitis which are the common organisms causing neonatal meningitis. Ampicillin is the preferred antibiotic to treat meningitis caused by these pathogens [29].
Transfer of ampicillin across the human placenta
The concentration of ampicillin becomes similar between the foetal and maternal plasma 90 min after ampicillin administration [30]. Ampicillin was administered to 103 women at delivery and ampicillin concentrations are similar in the cord and maternal plasma [31]. Sixty pregnant women were treated with ampicillin sodium at a dose of 500 mg and serum ampicillin sodium concentration ranges from 0.30+0.02 to 7.88+0.25 µg/ml in the maternal serum and the peak concentration in the cord serum is 2.37+0.16 µg/ml. These results are consistent with the view that ampicillin freely crosses the human placenta [32].
Migration of ampicillin into the breast-milk
In 3 lactating women, who received ampicillin at a dose of 2 grams daily, the milk concentration of ampicillin ranged from 0.3 to 0.9 µg/ml. In 3 lactating women, who received ampicillin at a dose of 4 grams daily, the ampicillin milk concentration ranged from 0.4 to 0.9 µg/ml. In all cases, the peak milk concentration of ampicillin occurred 3 hours after dosing [33]. These results show that ampicillin migrates into the breast-milk in significant amounts.
Discussion
Ampicillin is active against Streptococcus pyogenes, many strains of Streptococcus pneumoniae, and Haemophilus influenzae, meningococci, and Listeria monocytogenes. Ampicillin is used to treat sinusitis, otitis media, acute exacerbations of chronic bronchitis, and epiglottitis caused by sensitive organisms. Ampicillin may be administered intravenously, intramuscularly, or orally and following oral dosing ampicillin is well absorbed [1]. Ampicillin is effective and safe in treating infections caused by Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis [2] and high-dose of ampicillin/sulbactam is efficacy and safe in treating multidrug-resistant Acineto-bacter baumannii [3]. Ampicillin/sulbactam is active as amoxicillin/clavulanate in treating upper respiratory-tract infections [4]. Ampicillin/sulbactam and cefuroxime are efficacy and safe in treating serious skin and skin structure infections in paediatric patients. [5]. Ampicillin/sulbactam is efficacy and safe in treating many life-threatening infections in paediatric patients [6], and diabetic foot infections [7]. Ampicillin and sulbactam penetrate into different tissues in significant amounts [8], and in the prostate [9]. Khat chewing reduces the oral bioavailability of ampicillin [10]. Following intravenous administration of ampicillin, the concentration of ampicillin into the nasal polypus is 1.67 µg/gram [11]. The pharmacokinetics of ampicillin and sulbactam have been studied in healthy subjects and in patients with acute kidney injury subjected to extended or intermittent dialysis [12]. The elimination half-life of ampicillin is 1.41 hours in healthy subjects and it is longer in patients with acute kidney injury subjected to extended or intermittent dialysis. The elimination half-life of sulbactam is 1.73 hours in healthy subjects and it is longer in patients with acute kidney injury subjected to extended or intermittent dialysis. The prophylaxis with ampicillin/sulbactam or ampicillin prevents infections in patients subjected to surgery [13-19]. Prophylaxis with ampicillin/sulbactam prevents infections in patients undergoing cardiovascular surgery [13], and in women subjected to Caesarean delivery [14]. Single-dose of ampicillin/sulbactam provides better prophylaxis than single-dose of ampicillin in preventing infections in women undergoing Caesarean delivery [15]. Prophylaxis with single-dose of ampicillin prevents infections during surgery more effectively than standard penicillin prophylaxis [16], prophylaxis with ampicillin/tinidazole or cefotaxime is equally effective in preventing infection during hysterectomy [17]. Prophylactic ampicillin, given at the dose of 1 gram twice daily orally, prevents chronic bronchitis [18], and prophylactic ampicillin prevents infections in patients undergoing gastric surgery [19]. Treatment of infections with ampicillin or with ampicillin/sulbactam has been extensively studied [20-26]. Ampicillin is effective as cefazolin or ceftriaxone in treating childhood community-acquired pneumonia [20]. Ampicillin/sulbactam treats aspiration-associated pulmonary infections [21]. Ampicillin-ceftriaxone treats orthopaedic infection caused by Enterococcus faecalis [22], and ampicillin/sulbactam effectively treats urinary-tract, skin, soft tissue, bones and joints, respiratory-tract, ear, nose, throat, intra-abdominal, obstetric, gynaecological infections, and septicaemia [23]. Five-hundred mg of ampicillin is effective as 250 mg of cefaclor in treating urinary-tract infections [24], ampicillin given at a dose of 100 mg/kg for 5 days is affective as amoxicillin, given at the same dose, in treatment of infection caused by Salmonella gastroenteritis [25]. Ampicillin, administered at a dose of 100 mg/kg for 5 days, treats the meningitis caused by Haemophilus influenzae type b as rifampin given at a dose of 20 mg/kg for 4 days [26]. The penetration of ampicillin into the cerebrospinal fluid has been reported in 3 studies [27-29]. Amoxicillin and ampicillin were administered intravenously at a dose of 33 mg/kg twice-daily and ampicillin produces higher concentrations than amoxicillin in the cerebrospinal fluid [27]. Ampicillin was administered intravenously at a dose of 50 to 60 mg/kg to patients with listeric meningitis, and about 40 min after dosing, ampicillin concentration in the cerebrospinal fluid ranges from 5 to 8 µg/ml. In a patient with renal failure and listeric meningitis, the concentration of ampicillin in the cerebrospinal fluid ranges between 60 and 130 µg/ml and ampicillin successfully treated listeric meningitis in all occasions [28]. Newborns with septicaemia, caused by Staphylococcus aureus, were randomized to receive tobramycin or ceftazidime or ampicillin and ampicillin reaches higher concentrations in the cerebrospinal fluid than tobramycin and ceftazidime. Ampicillin is the preferred antibiotic to treat meningitis caused by Streptococcus aureus, enterococci and Listeria meningitis which are the common bacteria causing neonatal meningitis [29]. The transfer of ampicillin across the human placenta has been reported in 3 studies [30-32] and ampicillin freely crosses the human placenta. Ampicillin migrates into the breast-milk in significant amounts [33].
In conclusion, ampicillin is an aminopenicillin thus ampicillin is a β-lactam antibiotic which is active against gram-positive and gram-negative organisms. As bacteria possessing β-lactamases destroy the β-lactam ring, ampicillin is co-formulated with sulbactam which is an inhibitor of β-lactamases and ampicillin/sulbactam markedly expands the antimicrobial spectrum of ampicillin. Ampicillin is used to treat sinusitis, acute exacerbations of chronic bronchitis, epiglottitis, urinary-tract infections, and bacterial meningitis caused by sensitive organisms. Ampicillin may be administered intravenously, intramuscularly or orally and after oral dosing ampicillin is well absorbed. The efficacy and safety of ampicillin and ampicillin/sulbactam have been reported. Ampicillin and sulbactam penetrate in different tissues in significant amounts. The pharmacokinetics of ampicillin and sulbactam have been studied in healthy subjects and in patients with acute kidney injury submitted to extended or intermittent dialysis. In healthy subjects the elimination half-life of ampicillin and sulbactam is 1.41 and 1.73 hours, respectively, and the ampicillin and sulbactam half-life is increased in patients with acute kidney injury submitted to extended or intermittent dialysis. The prophylaxis with ampicillin/sulbactam or ampicillin and the treatment with ampicillin/sulbactam or with ampicillin have been extensively studied. Ampicillin penetrates into the cerebrospinal fluid in significant amounts and ampicillin treat bacterial meningitis however ampicillin may become resistant to different pathogens.
References
- MacDougal C, Hilal-dandan LL, Knollmann BC. (2018) “Penicillins, Cephalosporin, and Other β-Lactam Antibiotics”. In The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, Brunton editors. 1023-1038. [Ref.]
- Kohno S, Tateda K, Mikamo H, Kadota JI, Niki Y, et al. (2015) Efficacy and safety of intravenous sulbactam/ampicillin 3 g 4 times daily in Japanese adults with moderate to severe community-acquired pneumonia: a multicenter, open-label, uncontrolled study. J Infect Chemother. 21(3): 182-188. [PubMed.]
- Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. (2008) Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 56(6): 432-436. [PubMed.]
- Ferreira JB, Rapoport PB, Sakano E, Kós AODA, Piltcher OB, et al. (2006) Efficacy and safety of Sultamicillin (Ampicillin/Sulbactan) and Amoxicillin/Clavulanic acid in the treatment of upper respiratory tract infections in adults--an open-label, multicentric, randomized trial. Braz J Otorhinolaryngol. 72(1): 104-111.' [PubMed.]
- Azimi PH, Barson WJ, Janner D, Swanson R. (1999) Efficacy and safety of ampicillin/sulbactam and cefuroxime in the treatment of serious skin and skin structure infections in pediatric patients. UNASYN Pediatric Study Group. Pediatr Infect Dis J. 18(7): 609-613. [PubMed.]
- Kanra G. (2002) Experience with ampicillin/sulbactam in severe infections. J Int Med Res. 30 (Suppl 1): 20A-30A. [PubMed.]
- Akova M, Ozcebe O, Güllü I, Unal S, Gür D, et al. (1996) Efficacy of sulbactam-ampicillin for the treatment of severe diabetic foot infections. J Chemother. 8(4): 284-289. [PubMed.]
- Klotz T, Braun M, Saleh AB, Orlovski M, Engelmann U. (1999) Penetration of a single infusion of ampicillin and sulbactam into prostatic tissue during transurethral prostatectomy. Int Urol Nephrol. 31(2): 203-209. [PubMed.]
- Wildfeuer A, Luckhaupt H, Springsklee M. (1991) Concentrations of ampicillin and sulbactam in serum and tissues of patients undergoing ENT surgery. Infection. 19(1): 58-60. [PubMed.]
- Attef OA, Ali AA, Ali HM. (1997) Effect of Khat chewing on the bioavailability of ampicillin and amoxicillin. J Antimicrob Chemother. 39(4): 523-525. [PubMed.]
- Jeppesen F, Illum P. (1972) Concentration of ampicillin in antral mucosa following administration of ampicillin sodium and pivampicillin. Acta Otolaryngol. 73(5): 428-432. [PubMed.]
- Lorenzen JM, Broll M, Kaever V, Burhenne H, Hafer C, et al. (2012) 1. Pharmacokinetics of ampicillin/sulbactam in critically ill patients with acute kidney injury undergoing extended dialysis. Clin J Am Soc Nephrol. 7(3): 385-390. [PubMed.]
- Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Yamamoto H, et al. (2016) The pharmacokinetics of ampicillin-sulbactam in anuric patients: dosing optimization for prophylaxis during cardiovascular surgery. Int J Clin Pharm. 38(4): 771-775. [PubMed.]
- Eleftherios ZE, Tsiodras S, Matalliotakis I, Giamarellou H, Kanellakopoulou K. (2010) Ampicillin/sulbactam versus cefuroxime as antimicrobial prophylaxis for cesarean delivery: a randomized study. BMC Infect Dis. 10: 341. [PubMed.]
- Rijhsinghani A, Savopoulos SE, Walters JK, Huggins G, Hibbs JR. (1995) Ampicillin/sulbactam versus ampicillin alone for cesarean section prophylaxis: a randomized double-blind trial. Am J Perinatol. 12(5): 322-324. [PubMed.]
- Reggiori A, Ravera M, Cocozza E, Andreata M, Mukasa F. (1996) Randomized study of antibiotic prophylaxis for general and gynaecological surgery from a single centre in rural Africa. Br J Surg. 83(3): 356-359. [PubMed.]
- McDonald PJ, Sanders T, Higgins G, Finlay-Jones L, Hakendorf M, et al. (1984) Antibiotic prophylaxis in hysterectomy--cefotaxime compared to ampicillin-tinidazole. J Antimicrob Chemother. 14: (Suppl B): 223-230. [Ref.]
- Hahn HH, MacGregor RR, Avent CK, Counts GW, Smith HE, et al. (1972) Ampicillin and tetracycline in the treatment and prophylaxis of chronic bronchitis. Antimicrob Agents Chemother. 2(1): 45-48. [PubMed.]
- Madsen P, Rasmussen F, Hansen OH. (1971) Wound infection prophylaxis with topical ampicillin (Pentrexyl R) in gastric surgery. Scand J Gastroenterol. 6(3): 237-240. [PubMed.]
- Betrosian AP, Frantzeskaki F, Xanthaki A, Georgiadis G. (2007) High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Scand J Infect Dis. 39(1): 38-43. [PubMed.]
- Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H. (2008) Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 36(1): 23-30. [PubMed.]
- Euba G, Lora-Tamayo J, Murillo O, Pedrero S, Cabo J, et al. (2009) Pilot study of ampicillin-ceftriaxone combination for treatment of orthopedic infections due to Enterococcus faecalis. Antimicrob Agents Chemother. 53(10): 4305-4310. [PubMed.]
- Campoli-Richards DM, Brogden RN. (1987) Sulbactam/ampicillin. A review of its antibacterial activity, pharmacokinetic properties, and therapeutic use. Drugs. 33(6): 577-609. [PubMed.]
- Baraff LJ, Ablon WD. (1984) Cefaclor versus ampicillin for outpatient treatment of urinary tract infections. Am J Emerg Med. 2(4): 327-330. [PubMed.]
- Nelson JD, Kusmiesz H, Jackson LH, Woodman E. (1980) Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics. 65(6): 1125-1130. [PubMed.]
- Gessert C, Granoff DM, Gilsdorf J. (1980) Comparison of rifampin and ampicillin in day care center contacts of Haemophilus influenzae type b disease. Pediatrics. 66(1): 1-4. [PubMed.]
- Clumeck N, Thys JP, Vanhoof R. Vanderlinden MP, Butzler JP, et al. (1978) Amoxicillin entry into human cerebrospinal fluid: comparison with ampicillin. Antimicrob Agents Chemother. 14(4): 531-532. [PubMed.]
- Iwarson SF, Svedhem A, Svensson R. (1978) Cerebrospinal fluid concentrations of ampicillin in listeric meningitis. J Antimicrob Chemother. 4(3): 229-232. [PubMed.]
- Tessin I, Trollfors B, Thiringer K, Thörn Z, Larsson P. (1989) Concentrations of ceftazidime, tobramycin and ampicillin in the cerebrospinal fluid of newborn infants. Eur J Pediatr. 148(7): 679-681. [PubMed.]
- Boréus LO. (1971) Placental transfer of ampicillin in man. Acta Pharmacol Toxicol (Copenh). 29 (Suppl 3): 250-254. [PubMed.]
- MacAulay MA, Abou-Sabe M, Charles D. (1966) Placental transfer of ampicillin. Am J Obstet Gynecol. 96(7): 943-950. [PubMed.]
- Creatsas G, Pavlatos M, Lolis D, Kaskarelis D. (1980) Ampicillin and gentamicin in the treatment of fetal intrauterine infections. J Perinat Med. 8(1): 13-18. [PubMed.]
- Pons G, Rey E. (1994) Passage of antibiotics in breast milk. Med Mal Infect. 24(1): 1088-1106. [Ref.]