>Corresponding Author : Iván González
>Article Type : Research Article
>Volume : 2 | Issue : 1
>Received Date : 12 Oct, 2022
>Accepted Date : 27 Oct, 2022
>Published Date : 31 Oct, 2022
>DOI : https://doi.org/10.54289/JCCP2200102
>Citation : Esguerra J, González I, Rojas F, Camargo J and Jimenez A. (2022) Effectiveness and Safety of Ocular Brachytherapy, Experience at The National Cancer Institute of Colombia, 2010-2018. J Cancer Cancer Prev 2(1): doi https://doi.org/10.54289/JCCP2200102
>Copyright : © 2021 Esguerra J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access | Full Text
1Radiation Oncology, Instituto Nacional de Cancerología, Calle 1 No. 9 -85 Bogotá DC, Colombia
2Ophthalmic Oncology, Instituto Nacional de Cancerología, Calle 1 No. 9 -85 Bogotá DC, Colombia
*Corresponding author: Iván González, Radiation Oncology, Instituto Nacional de Cancerología, Calle 1 No. 9 -85 Bogotá DC, Colombia
Summary
Introduction: The most frequent primary ocular tumors in adult and pediatric populations are melanoma and retinoblastoma, respectively. The treatment of these pathologies is a challenge for the treating physician, since they must balance the need for disease control and the preservation of the organ and its function, in the scenario of localized disease. This is usually achieved through a combination of local control therapies; however, in some scenarios, enucleation is the only treatment option available. Since its implementation, ocular brachytherapy has established itself as a therapeutic alternative that offers adequate local control while preserving the eyeball and some degree of visual function. This study seeks to describe the effectiveness and safety of ocular brachytherapy, in a consecutive series of patients treated at National Cancer Institute of Colombia.
Materials and methods: The present study is an observational, retrospective, case series analysis, conducted at the National Cancer Institute of Colombia. It included patients diagnosed with primary tumors of the eye, including ocular melanoma, retinoblastoma, and ocular hemangioma, who were treated with iodine-125 ocular brachytherapy between 2010 and 2018.
Results: The medical records of 58 patients were reviewed, who were treated with ocular brachytherapy at the National Cancer Institute of Colombia between January 1, 2010, and December 31, 2018. Thirty-six patients met the selection criteria. The predominant histological subtype was melanoma in 34 (94.4%) patients. The median radiation dose received was 85 Gy (ranging from 48 Gy to 100 Gy); 69.4 % of patients (n=25) had a complete response to treatment at the 6-month post-treatment evaluation. Of the total number of patients analyzed at the 60-month follow-up, 6 (16.6%) presented distant metastases, with hepatic (n=2), lumbar spine (n=1), supraclavicular ganglion (n=1), pulmonary (n=1), and hepatic and pulmonary (n=1) location. The median metastasis-free time was 33.5 months. Three of these patients died of oncological causes. Overall survival at 36 and 60 months was 96.6% (95% CI: 90.1-100) and 89.4% (95% CI: 78.7-100), respectively. Unstratified disease-free survival at 36 and 60 months was 87.0% (95% CI: 75.9-99.8) and 77.5% (95% CI: 62.4-96.2), respectively. A total of three patients required enucleation, one of them due to treatment-associated toxicity.
Conclusion: Ocular brachytherapy is a safe and effective treatment strategy, which allows for preserving the eyeball and vision in the medium and short term while allowing adequate tumor control in the eyeball.
Keywords: Ocular Brachytherapy; Plaque Radiotherapy; Retinoblastoma; Ocular Melanoma; Survival; Local Control.
Abbreviations: UM: Uveal Melanoma, SEER: Surveillance, Epidemiology, and End Results, ABS: American Brachytherapy Society, COMS: Collaborative Ocular Melanoma Study
Introduction
Melanoma is a malignant tumor that arises from melanocytes located in various anatomical locations, including the skin, mucous membranes (nasal, oropharyngeal, pulmonary, gastrointestinal, vaginal, anal/rectal, urinary tract mucosa), ocular region (uvea, conjunctiva, eyelid, orbit), and rarely from unknown primary sites [1]. Uveal melanoma (UM) is the most common primary intraocular neoplasm in adults. According to reports from the US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program database, between 1973 and 2008, the average age-adjusted incidence of UM is 5.1 cases per million population per year [2]. Among ocular melanomas, approximately 83% arise from the uvea, 5% from the conjunctiva, and 10% from other sites. The most common site for UM is the choroid [1,2]. On the other hand, retinoblastoma is the most common ocular malignancy in childhood, with an incidence of approximately 1 per 15,000 to 18,000 live births. There is no great variation between races and genders, and it is estimated that there are approximately 5,000 new cases worldwide each year [3]. In 60% of cases, the disease is unilateral, with a median age at diagnosis of two years; 15% of these cases are hereditary. Approximately 40% of cases are bilateral and less than 5% trilateral, with a median age of one year at diagnosis [4].
Both conditions represent a therapeutic challenge for the treating physician since they imply the need for disease control against the preservation of vision and the eyeball. Currently, in diseases located in the eyeball, organ-sparing therapies (intravenous chemotherapy, intra-arterial chemotherapy, intravitreal chemotherapy, cryotherapy, transpupillary thermotherapy, laser therapy, external beam radiotherapy, brachytherapy) are favored over enucleation, which in most cases requires interventions by multidisciplinary groups, including oncologic ophthalmology, clinical oncology, and radiation oncology.
The use of brachytherapy for the treatment of an intraocular tumor was first reported in 1930 by Foster Moore, in a patient diagnosed with left melanocytic sarcoma, who refused enucleation given that his contralateral eye had little vision. The use of brachytherapy is common in these pathologies. In this report, two applications with radon seeds of 1 and 5 millicuries were used on a 0.5 mm platinum plaque, which was removed 14 and 10 days after insertion, respectively, achieving symptomatic control and tumor volume reduction [5]. The use of “seeds” or sources of 60-Co, 106-Ru, 125-I, 103-Pd, 90-Sr, and 131-Cs has been described [6].
Modern brachytherapy applicators or “plaques” are gold shells that facilitate the placement of seeds in pre-established distributions that are configured on a case-by-case basis (Image 1).
Nowadays, brachytherapy has been consolidated as an alternative to enucleation in the management of diseases such as retinoblastoma and melanoma, and it is currently considered an effective method to preserve the organ and vision in the treatment of patients with intraocular tumors. Current ABS (American Brachytherapy Society) indications for ocular brachytherapy include any patient with a clinical diagnosis of melanoma, without the need for histopathological confirmation, with T1 to T4d tumors, who still retain some vision, have no extraocular extension, or pain due to the tumor [6]. Likewise, brachytherapy is an alternative treatment for retinoblastoma, especially in the setting of bilateral disease, local recurrence or failure to control with other therapies (cryotherapy, intra-arterial or ophthalmic chemotherapy, laser, or cryotherapy) [7].
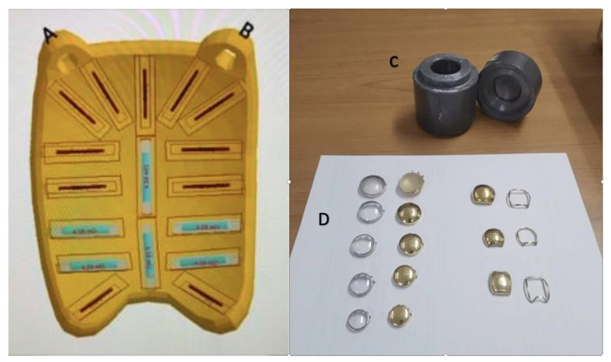
Image 1: Predetermined source position distribution within the brachytherapy plaque. A and B correspond to plaque fixation sites. C. Lead container for source disposal. D. Size and shape options for plaques and their respective dummy plaques. 28 29 237x138mm (300 x 300 DPI)
Surgical management with enucleation was the primary treatment for UM for more than 100 years. The Collaborative Ocular Melanoma Study (COMS) confirmed in2001 that globe-preserving episcleral brachytherapy was a safe and effective alternative in the treatment of UM and showed no differences in survival with enucleation [8]. This study included 1,317 patients diagnosed with unilateral choroidal melanoma, with basal diameters not exceeding 16 mm and apical heights of 2.5 to 10 mm; they were randomized to be treated with enucleation or ocular brachytherapy. The prescribed dose was 85 Gy to tumor apex and the isotope of choice was I-125 (Iodine 125). After 12 years of follow-up to 515 patients, 231 (45%) were alive and clinically cancer-free. In both treatment groups, all-cause mortality rates at 5 and 10 years were 19% and 35%, respectively. Death rates at 5, 10, and 12 years with histopathologically confirmed melanoma metastases were 10, 18, and 21% in the brachytherapy group with 125-I, and 11, 17, and 17% in the enucleation group [9]. In this group, the mean visual acuity at baseline in the compromised eye was 20/32 (70% of the eyes with 20/40 or better and 10% with 20/200 or worse visual acuity). Three years after brachytherapy, the mean visual acuity was 20/125, with 34% with 20/40 or better, and 45% with 20/200 or worse visual acuity, including eyes that were enucleated within 3 years of treatment. Vision loss was related to the administered dose, tumor size, depth, and shape (tumors that were not dome-shaped), history of diabetes, proximity of the tumor to the macula, or history of retinal detachment associated with the tumor [10].
In an analysis of the National Cancer Database, Messer et al. describe 7,096 patients diagnosed with UM; 5,501 patients received brachytherapy and 1,595 were treated with enucleation. In this report, the 5-year overall survival for small tumors (diameter < 18 mm, height < 2.5 mm) was 87% and 64%; for medium tumors (diameter < 18 mm, height 2.5-10 mm), it was 77% and 57%, and for large tumors (diameter > 18 mm, height > 10 mm) it was 68% and 46% for brachytherapy and enucleation, respectively (p<0.001). Older age, more comorbidities, extraocular extension, ciliary body invasion, and larger size were negative prognostic factors for survival. Brachytherapy was an independent positive prognostic factor for overall survival [11].
Brachytherapy in retinoblastoma has been compared to external beam radiotherapy (more commonly used) as it offers the advantage of being administered completely within 2 to 4 days, with the disadvantage of requiring surgical plaque implantation and removal. Generally, I-125 is used at a dose of 35 to 45 Gy at the tumor apex, administered 1 to 2 months after intravenous chemotherapy, to minimize side effects [12]. In a review of 400 patients with retinoblastoma, Shields et al. describe 103 tumors in 103 eyes treated with ocular brachytherapy. Tumors ranged in diameter between 1 to 16 mm and in thickness between 1 to 8 mm. Thirty-one patients received initial treatment with brachytherapy and the remaining 72 were treated after failure of other therapeutic alternatives. All patients responded to brachytherapy with tumor regression. During follow-up (mean = 38 months), 89 patients (87%) had tumor regression and 13 (13%) showed tumor recurrence, which occurred in a mean interval of five months [13]. The clinical advantages of ocular brachytherapy over external radiotherapy in the treatment of retinoblastomas include a lower risk of orbital and facial hypoplasia and a lower risk of presenting second primary tumors because of treatment [12].
Follow-up after brachytherapy for both ocular melanoma and retinoblastoma aims to specifically evaluate local control, complications, organ preservation, and presentation of systemic disease. This is usually done every 3 to 6 months, depending on the likelihood of secondary complications and clinical behavior of the disease.
Complications of ocular brachytherapy usually occur within the first 3 years and are more frequent in larger tumors; in a series of 354 patients with tumors thicker than 8 mm, Shields et al. describe proliferative retinopathy in 25%, maculopathy in 24%, papillopathy in 22%, cataracts in 66%, neovascular glaucoma in 21%, vitreous hemorrhage in 23%, and scleral necrosis in 7% of patients presenting with adverse events. Enucleation was necessary in 24% of patients at 5-year follow-up and in 34% at 10 years [14].
The National Cancer Institute of Colombia (INC, for its acronym in Spanish) has been using this therapy since 2006 and is the only institution at the national level that continues to have this treatment available. The absence of local and regional data regarding the benefit offered by ocular brachytherapy in the treatment of ocular melanoma and retinoblastoma opens the opportunity to provide local data regarding response to treatment in this group of pathologies.
This work aims to describe the effectiveness and adverse effects associated with ocular brachytherapy at the INC, during the period from 2010 to 2018, in the treatment of patients with primary ocular tumors. The results of this study will allow designing improvement plans aimed at optimizing the resources invested in treatments, making ocular brachytherapy visible as a valid, safe, and effective treatment alternative available in Colombia, as well as establishing the bases for clinical studies and the implementation of ocular brachytherapy, to improve the clinical outcomes of patients treated in similar oncological institutions.
Materials and methods
The present study is an observational, descriptive, retrospective, case series analysis, carried out at the INC. It included patients with diagnoses of primary ocular tumors, without metastases at the time of treatment, who received ocular brachytherapy with curative intent between 2010 and 2018. Patients without histological confirmation of the tumor were excluded, as well as patients without appropriate clinical follow-up for at least one year, and patients where complete data on the therapies administered (primary therapy and adjuvant) were not available. Data collection for the development of the study was based on information from institutional clinical registries. The main objective was to describe the effectiveness and safety of treatment with ocular brachytherapy in the Department of Radiation Oncology of the INC during the period from 2010 to 2018. Clinical outcomes such as local disease control, distant metastasis, disease-free survival, vision preservation, and treatment-associated adverse effects reported in medical records were considered. All patients registered in the database of brachytherapy procedures at the Department of Medical Physics of the INC were taken as a reference. Subsequently, data were filtered according to year and diagnosis of interest; medical records were reviewed applying the inclusion and exclusion criteria, and those patients who were candidates for inclusion were tabulated in a virtual data collection form, on the RedCap platform, administered by the INC’s statistics group. Finally, data were manually verified by the research monitoring group, to guarantee information confidentiality and veracity. The information collected was analyzed using R-Project version 4.0.3 (free license; descriptive frequencies were established, mainly median, mean, and absolute percentages for each outcome.
Results
Population
Medical records of 58 patients were reviewed, who were treated with ocular brachytherapy at the INC between January 1, 2016, and December 31, 2019. Thirty-six patients met the selection criteria; patients were excluded mostly due to incomplete follow-up. Forty-four variables were completed in the data capture tool for each patient. Age range was from 5 to 91 years, with a median of 60.5 years (interquartile range: 23); 50% of the patients were female. The predominant histological subtype was melanoma in 34 patients (94.4%). Table 1 presents the main sociodemographic, clinical, and treatment characteristics for this group of patients.
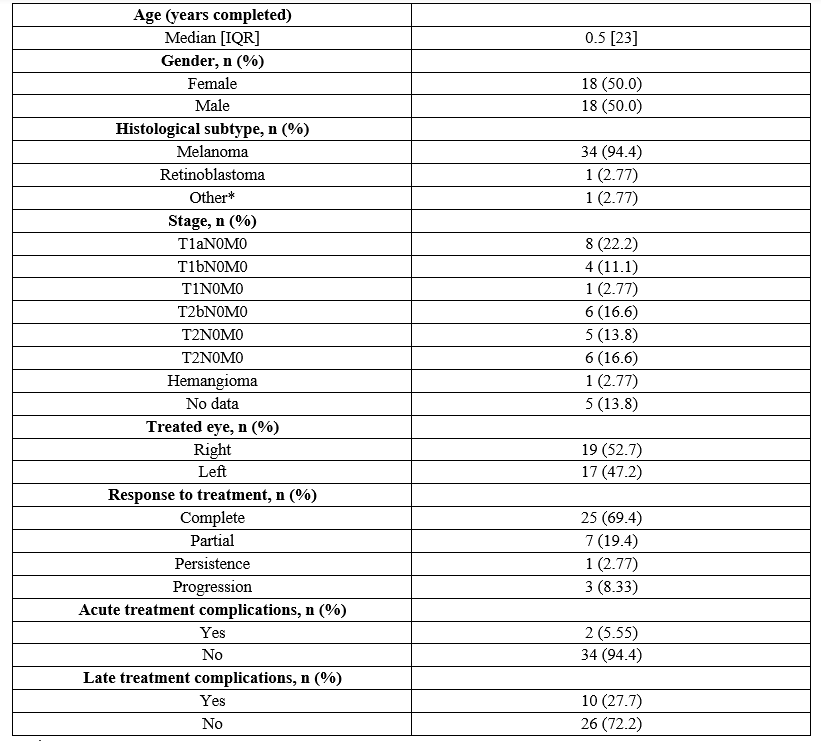
*Hemangioma
IQR: Interquartile range
Table 1: Sociodemographic, histopathological, and treatment characteristics in patients diagnosed with ocular tumors at the INC.
The most frequent tumor location was temporal (15 patients), while the other locations did not show a localization trend. Thirty-four patients were diagnosed with melanoma, one patient was diagnosed with retinoblastoma, and another one with hemangioma.
Treatment with radiotherapy
In the group of patients analyzed, the most frequent stage was T1aN0M0 (n=8, 22.2%). The median radiation dose received was 85 Gy (which ranged from 48 Gy to 100 Gy). This variation corresponds to a patient diagnosed with retinoblastoma, who received a dose of 4000 cGy to the tumor apex. None of the patients received external radiotherapy according to medical records. Regarding additional therapies, 58.3% (n=21) received additional treatments: transpupillary thermotherapy (n=15, 41.55%), sandwich therapy-laser (n=2, 5.54%), and carboplatin and etoposide + subconjunctival carboplatin + laser photocoagulation (n=1, 2.77%).
Oncological control and adverse effects
69.4% (n=25) of patients had a complete response to treatment at 6-month follow-up. Of the total number of patients analyzed, 6 patients (16.6 %) presented distant metastases, with hepatic (n=2), lumbar spine (n=1), supraclavicular ganglion (n=1), pulmonary (n=1), and hepatic and pulmonary (n=1) location. The median metastasis-free time, defined as the time between the date of treatment and the date of diagnosis of metastatic lesions, was 33.5 months with a minimum of 0.52 months and a maximum of 82.6 months. Three of these patients died during follow-up, with time between death and diagnosis of metastasis being 2.36, 2.46, and 38.4 months, respectively. With respect to acute complications, only 5.55% (n=2) presented them, which were conjunctival hyperemia, a patient who received a dose of 85 Gy to the apex, with thermotherapy as an additional treatment for a partial response to treatment; and moderate conjunctivitis with doses of 80 Gy without additional treatment and with partial response to treatment. Regarding late complications, 27.7% (n=10) presented some type, which included cataract (n=3), dry eye (n=2), radiotherapy retinopathy (n=1), painful red eye and secondary neovascular glaucoma requiring enucleation (n=1), painful eye (n=1), and burning and dry eye (n=2).
In total, enucleation was performed in 11.8% (n=5) of patients. Two of these patients had a complete response to treatment and the rest had disease progression after treatment.
For overall survival, follow-up time was defined as the time between the date of death or the date of last contact and the date of treatment. In the case of disease-free survival, it corresponded to time between the date of relapse, date of death or date of last contact, and the date of treatment. The Kaplan-Meier estimator was used to calculate the 36- and 60-month survival curves. Survival curves at total follow-up are presented in Figure 1.
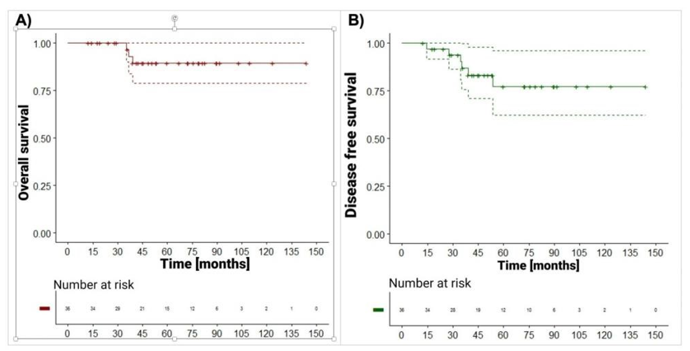
Figure 1: Predetermined source position distribution within the brachytherapy plaque. A and B correspond to plaque fixation sites. C. Lead container for source disposal. D. Size and shape options for plaques and their respective dummy plaques. 28 29 237x138mm (300 x 300 DPI)
Overall survival
Of the 36 patients diagnosed with ocular tumors, 3 patients died due to oncological causes. The median follow-up time was 52.1 months (12.4 to 144 months). Overall survival at 36 and 60 months was 96.6% (95% CI: 90.1-100) and 89.4% (95% CI: 78.7-100), respectively (Figure 1, panel A).
Disease-free survival
Six patients had relapse and died due to oncological causes, of which 3 only had relapse, 2 patients only died, and 1 patient had relapse and died (the difference in months between the date of death and relapse was 3.52). Unstratified disease-free survival at 36 and 60 months was 87.0% (95% CI: 75.9-99.8) and 77.5% (95% CI: 62.4-96.2), respectively (Figure 1 panel B).
Preservation of vision
Regarding visual acuity assessment before treatment with brachytherapy, 21 patients (58.17%) had vision greater than 20/200, with an average of 20/50. Of the remainder, 13 patients had vision of 20/200 or worse, and 2 patients had no visual acuity assessment reported in their medical history. After brachytherapy, at 6-month follow-up, 18 patients had vision greater than 20/200, with an average vision of 20/60, 12 patients had vision of 20/200 or worse, 2 patients had no visual acuity measurement at follow-up, and 2 patients had no follow-up. At the one-year post-brachytherapy assessment, 18 patients had vision greater than 20/200, with an average of 20/45; 12 patients had vision of 20/200 or worse, 2 patients had no visual acuity measurement at follow-up, and 2 patients had no follow-up. At 18 months, 14 patients retained vision greater than 20/200, with an average of 20/47. Fourteen patients had vision of 20/200 or worse, 5 patients had no visual acuity assessment at follow-up, and 3 patients had no follow-up. Finally, in the clinical control at 3 and 5 years after the ocular brachytherapy procedure, 4 and 3 patients retained a vision greater than 20/200, respectively. Three patients underwent enucleation within 5 years of brachytherapy due to disease progression, 8 patients had a visual acuity measurement equal to or less than 20/200, 11 patients had no report of visual acuity, and 3 patients had no follow-up.
Discussion
The most common primary ocular tumors in the adult and pediatric populations are melanoma and retinoblastoma, respectively. The treatment of these pathologies is a challenge for the treating physician since they must balance the need for disease control against the preservation of the organ and its function, especially in the scenario of localized disease. This is usually achieved through a combination of local control therapies and is generally preferred over enucleation.
Although eyeball preservation is usually achieved with I-125 plaque brachytherapy, most patients experience decreased visual function secondary to radiation complications [15]. This visual impairment was evidenced in our case series mainly at 3-year follow-up and is consistent with data reported in the literature.
Specialized groups such as the Alberta Ocular Brachytherapy Program in Canada describe a complete response rate of 62.5%; functionality during treatment and impaired vision in 52% of patients; alteration in reading ability in 60%, and the ability to perform daily care in 20.8% [16].
The data obtained show an adequate local control in the short term with only 3 patients undergoing enucleation; however, it is worth noting that since it was a retrospective study, visual acuity measurement and post-treatment follow-up was not as strict as what is obtained in a prospective study. Similarly, no deaths resulting from toxicity secondary to treatment were found and only one patient required enucleation due to non-oncological causes.
Based on the information found in our series, it is possible to confirm an adequate effectiveness, with a favorable adverse event profile, while also preserving the eyeball in a large part of treated patients, as well as vision in the short term, and in a smaller proportion in the long term. It is worth noting, however, that the comparison therapy in most of the mentioned scenarios would be enucleation as primary treatment.
Hopefully, the results of this study will serve as a basis for developing more extensive studies, which will allow studying in depth the benefits of this treatment alternative, disseminating the advantages of ocular brachytherapy to preserve the eyeball and vision in the short and, to a lesser extent, the long term, and encouraging other institutions to implement programs aimed at the treatment of ocular pathologies with this therapeutic modality.
Conflict of interest: The authors of this article declare that they have no conflict of interests in the preparation of the manuscript or in the interpretation of the results.
Funding sources: This article was developed with the authors’ own resources and with the human capital available at the departments involved of the National Institute of Cancerology. No external funding sources were used.
Acknowledgments: To the group of medical physics, Medical Residents in Radiation Oncology and supporting staff of Instituto National de Cancerología for their collaboration and cooperation.
Author contributions: José Esguerra – conceptualization and paper review, Iván González performed writing, editing and submission, Fernando Rojas and Jennifer Camargo conceptualization and paper review, Adriana Jimenez performed writing data collection.
References
- Kaliki S and Shields CL. (2017) Uveal Melanoma: Relatively Rare but Deadly Cancer. Eye. 31(2): 241-257. [PubMed.]
- Singh AD, Turell ME and Topham AK. (2011) Uveal Melanoma: Trends in Incidence, Treatment, and Survival. Ophthalmology. 118(9): 1881-1885. [PubMed.]
- Rao R and Honavar SG. (2017) Retinoblastoma. Indian J Pediatr. 84(12): 937-944. [PubMed.]
- Aerts I, Rouic LLL, Gauthier-Villars M, et al. (2006) Retinoblastoma. Orphanet J Rare Dis. 1(1): 31. [Ref.]
- Moore RF. (1930) Choroidal Sarcoma Treated by The Intraocular Insertion of Radon Seeds. British Journal of Ophthalmology. 14(4): 145-152. [PubMed.]
- Simpson ER, Gallie B, Laperrierre N, et al. (2014) The American Brachytherapy Society Consensus Guidelines for Plaque Brachytherapy of Uveal Melanoma and Retinoblastoma. Brachytherapy. 13(1): 1-14. [PubMed.]
- Francis JH, Barker CA, Wolden SL, et al. (2013) Salvage/Adjuvant Brachytherapy After Ophthalmic Artery Chemosurgery for Intraocular Retinoblastoma. International Journal of Radiation Oncology*Biology*Physics. 87(3): 517-523. [Ref.]
- Brewington BY, Shao YF, Davidorf FH, et al. (2018) Brachytherapy for Patients with Uveal Melanoma: Historical Perspectives and Future Treatment Directions. OPTH Volume. 12: 925-934. [Ref.]
- Collaborative Ocular Melanoma Study Group (2006) The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: V. Twelve-Year Mortality Rates and Prognostic Factors: COMS Report No. 28. Arch Ophthalmol. 124(12): 1684. [PubMed.]
- Melia BH, Abramson DH, Albert DM, Boldt HC, Earle JD, et al. (2001) Collaborative Ocular Melanoma Study (COMS) Randomized Trial of I-125 Brachytherapy for Medium Choroidal Melanoma I. Visual Acuity after 3 Years COMS Report No. 16. Ophthalmology. 108(2): 348-366. [PubMed.]
- Messer J, Zuhour R, Haque W, et al. (2020) Eye Plaque Brachytherapy versus Enucleation for Ocular Melanoma: An Analysis from the National Cancer Database. jcb. 12(4): 303-310. [PubMed.]
- Ancona-Lezama D, Dalvin L and Shields C. (2020) Modern Treatment of Retinoblastoma: A 2020 Review. Indian J Ophthalmol. 68(11): 2356-2365. [Ref.]
- Shields CL, Shields JA, Minelli S, et al. (1993) Regression of Retinoblastoma After Plaque Radiotherapy. American Journal of Ophthalmology. 115(2): 181-187. [PubMed.]
- Shields CL, Naseripour M, Cater J, et al. (2002) Plaque Radiotherapy for Large Posterior Uveal Melanomas (≥ 8-Mm Thick) in 354 Consecutive Patients 11Presented in Part at the Annual Meeting of the American Academy of Ophthalmology, October 2002. Ophthalmology. 109(10): 1838-1849. [PubMed.]
- Wen JC, Oliver SC and McCannel TA. (2009) Ocular Complications Following I-125 Brachytherapy for Choroidal Melanoma. Eye. 23(6): 1254-1268. [PubMed.]
- Read W, Crump RT and Weis E. (2016) The Alberta Ocular Brachytherapy Program: Utilization of Patient Feedback to Guide Improvements. Journal of Medical Imaging and Radiation Sciences. 47(4): 349-355. [PubMed.]