>Corresponding Author : Anubha Bajaj
>Article Type : Complimentary Article
>Volume : 1 | Issue : 1
>Received Date : 12 November, 2021
>Accepted Date : 19 November, 2021
>Published Date : 22 November, 2021
>DOI : https://doi.org/10.54289/JCCP2100102
>Citation : Bajaj A (2021) What Are the Markers That Predict the Development of Having Cancer in the Future Without Laboratory or Radiological Tests? J Cancer Cancer Prev 1(1). doi https://doi.org/10.54289/JCCP2100102
>Copyright : © 2021 Bajaj A . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Complimentary Article | Open Access | Full Text
Consultant Histopathologist, New Delhi, India
*Corresponding author: Anubha Bajaj, Consultant Histopathologist, New Delhi, India
Preface
Sebaceous glands configure normal cutaneous adnexal structures wherein sebaceous glands discerned within the oral cavity are nomenclated as Fordyce's granules (FGs) or heterotopic or ectopic sebaceous glands. Fordyce's granules appear identical to normal cutaneous adnexal sebaceous glands where an estimated 50% of sebum is constituted of triglycerides
Sebaceous glands within the oral cavity were initially described by Kölliker in 1861 and nomenclated by Fordyce in 1896. The granules are contemplated as aggregates of normal sebaceous glands superimposed with an intact mucosal epithelium.
Fordyce’s granules are denominated as a phenotypically normal variation or ectopic oral mucosal sebaceous glands devoid of hair follicles. The glands are discernible to the naked eye and situated within the oral or genital mucosa. Fordyce’s granules are additionally designated as Fordyce’s spots.
Lipid composition of Fordyce’s granules is identical to lipids incorporating cutaneous sebaceous glands. Clinical discernment is usually adequate although cogent tissue sampling may be mandated for identifying hyperplastic or nodular lesions.
However, individuals with increased quantities of Fordyce’s granules necessitate appropriate referral and medical supervision in order to categorize risk factors pertaining to cardiovascular disorders.
Disease Characteristics
An estimated 80% of adult population exhibits Fordyce’s granules. Possibly, the glands are present at birth or may enlarge and are discerned upon puberty although no age of disease incrimination is exempt [1,2].
Generally, Fordyce’s granules achieve prominence following puberty. A definitive racial or gender predilection is absent although a mild male predominance may be exemplified [2,3].
Fordyce’s granules may infrequently (5%) implicate singular cheek or buccal mucosa. Fordyce’s granules can emerge as undetectable spots or as clusters of exceeding > 100 glands. Nevertheless, density of Fordyce’s granules diminish with advancing age and peak age of disease occurrence is observed within 30 years to 50 years [2,3].
Fordyce’s granules are frequently delineated in non-smokers. Smoking may obscure the presence oral Fordyce’s granules, possibly due to a thickened oral epithelium and enhanced production of epithelial melanin occurring within chronic smokers [2,3].
A direct proportion is observed between density of Fordyce’s granules and values of serum lipids, especially with Fordyce’s granules exceeding ≥ 100 spots per individual [2,3].
Subjects with elevated lipids are associated with enhanced quantification of Fordyce’s granules, especially upon bilateral buccal mucosa and vermilion border of lips [2,3].
In contrast, individuals with few, unilateral, buccal Fordyce’s granules or isolated labial lesions may be associated with normal serum lipids. Hyperlipidaemia delineated in individuals beyond > 50 years is concurrent with increased density of Fordyce’s granules [2,3]. Fordyce’s granules are non infectious and do not manifest as a sexually transmitted disease. Implicated sebaceous glands are essentially normal although malignant metamorphosis is exceptional and documented [2,3]. Fordyce’s granules may be associated with Muir-Torre syndrome and Lynch syndrome which exhibit defective deoxyribonucleic acid (DNA) mismatch repair (MMR) proteins with consequent microsatellite instability [2,3].
Generally, Fordyce’s granules appear upon unilateral or bilateral buccal mucosa, vermilion border of upper lip, mandibular retro-molar pad and tonsillar area. Lesions may arise upon the genital region especially areolae, glans penis and labia minora [4,5].
Fordyce’s granules may be categorized pertaining to location as
• G1 with an absence of Fordyce’s granules
• G2 demonstrating Fordyce’s granules upon vermilion border of lips
• G3 exemplifying Fordyce’s granules upon unilateral buccal mucosa
• G4 enunciating Fordyce’s granules upon bilateral buccal mucosa
• G5 delineating Fordyce ‘s granules upon bilateral buccal mucosa and vermilion border of lips
• G6 exhibiting Fordyce’s granules upon unilateral buccal mucosa and vermilion border of lips [4,5].
It is posited that elevated lipids may engender Fordyce’s granules due to augmentation of lipid content within inconspicuous sebaceous glands or as a de novo lipid constituent amalgamated during cellular differentiation with consequently enhanced oral Fordyce’s granules.
Alternatively, Fordyce’s granules may emerge as congenital lesions or may be contemplated as a benign sebaceous hamartoma [4,5].
Clinical Elucidation
Fordyce’s granules emerge as miniature, mildly elevated, yellowish or whitish papules of magnitude varying from 1 millimetre to 5 millimetres and are confined to buccal mucosa and vermilion border of lips. Genital spots located within the glans penis or shaft of penis are scripted as Tyson’s glands. Vulva or vagina of females may demonstrate Fordyce’s granules. Ectopic sebaceous glands confined to breast areolae are enunciated as Montgomery’s glands [4,5].
Asymptomatic, ectopic sebaceous glands confined to the oral cavity or vermilion border of lips appear as hyperplastic, yellow or white papules or nodules of variable dimension may simulate a floret pattern. Fordyce’s granules may last a lifetime [4,5].
Fordyce’s granules may emerge as a solitary lesion or frequently depict a cluster of around 100 glands. The granules are conveniently observed upon stretching of incriminated cutaneous surfaces [4,5].
Histological Elucidation
Upon microscopic examination, mature sebaceous glands appear aggregated subjacent to mucosal epithelium. The visible sebaceous glands appear in continuum with superimposed cutaneous surface and are devoid of associated hair follicles [6,7]. Nevertheless, a ductal communication with superficial epidermis may be absent [6,7].
The morphological countenance is akin to normal cutaneous adnexal sebaceous glands. Superimposed stratified squamous epithelium demonstrates parakeratosis and exhibits subjacent lobules of mature sebaceous glands [6,7].
Upon immunostaining, Fordyce’s granules delineate a preservation of mismatch repair (MMR) proteins [6,7].
Fordyce’s granules require a segregation from conditions which appear identical on morphology such as sexually transmitted diseases with incrimination of the genital region [7,8].
Upon immunostaining, Fordyce’s granules delineate a preservation of mismatch repair (MMR) proteins [6,7].
Fordyce’s granules require a segregation from conditions which appear identical on morphology such as sexually transmitted diseases with incrimination of the genital region [7,8].
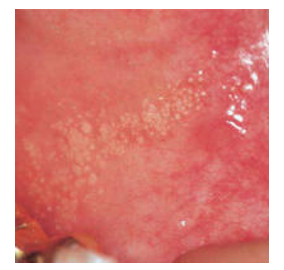
Figure 1: Fordyce's granules enunciating miniature, elevated yellowish or whitish papules upon the oral mucosa (9).
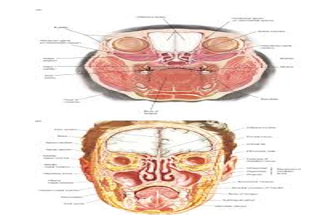
Figure 2: Fordyce's granules demonstrating diverse sites of emergence within the oral mucosa (10).
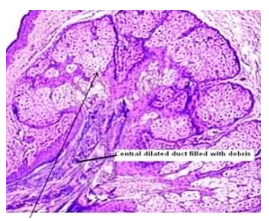
Figure 3: Fordyce's granules exhibiting enlarged sebaceous glands imbued with lipids and a superimposed stratified squamous epithelium (11).
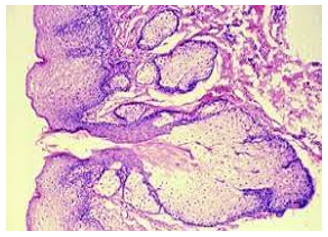
Figure 4: Fordyce's granules exemplifying numerous sebaceous lobules incorporated with lipids and an enveloping acanthotic stratified squamous epithelium (12).
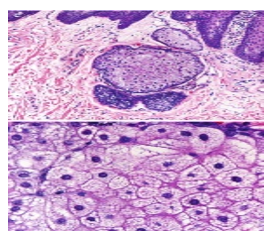
Figure 5: Fordyce's granules exhibiting enlarged sebaceous glands with lipid laden lobules and a superficial hyperkeratotic epidermal layer (13).
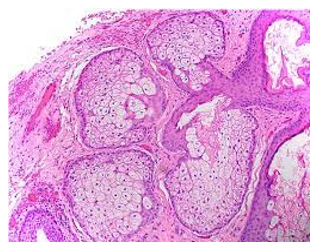
Figure 6: Fordyce's granules delineating several lobulated sebaceous glands with lipid-rich cells and an enveloping fibrotic stroma (14).
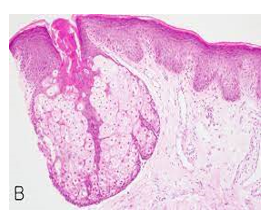
Figure 7: Fordyce's granules depicting an enlarged, lobulated sebaceous gland with a superimposed hyperkeratotic stratified squamous epithelium (15).
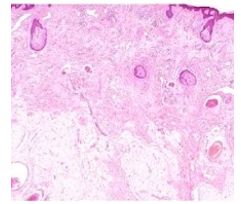
Figure 8: Fordyce's granules displaying several miniature sebaceous glands composed of lipid- rich cellular lobules and a minimally fibrotic stroma (16).
Investigative Assay
Fordyce’s granules can be diagnosed upon a precise clinical evaluation. Occasionally, tissue sampling of incriminated cutaneous surface may be required for histological concordance, especially with hyperplastic or nodular lesions [7,8].
Therapeutic Options
Fordyce’s granules may raise cosmetic concerns in certain individuals. Squeezing or compression of the spots is not recommended [7,8].
Electrosurgical techniques and vaporising carbon dioxide (CO2) laser may be beneficially employed to eradicate Fordyce’s granules [7,8].
References
- Halperin V, Kolas S, et al. (1953) The occurrence of Fordyce spots, benign migratory glossitis, median rhomboid glossitis, and fissured tongue in 2,478 dental patients” Oral Surg Oral Med Oral Pathol. 6: 1072–1077. [PubMed.]
- Gaballah KY, Rahimi I. (2014) Can presence of oral Fordyce's granules serve as a marker for hyperlipidemia?” Dent Res J (Isfahan). 11(5): 553-558. [Ref.]
- Ryu SI, Jeong JY, et al. (2021) Fordyce Spots Treated by an Intralesional Insulated Microneedle Radiofrequency Device” Dermatol Surg. 47(7): 1021-1022. [PubMed.]
- Poizeau F, Plantier F, et al. (2021) Vulvar Fordyce adenitis: A cohort of 45 women. Ann Dermatol Venereol”. 30: S0151-9638(21)00039-9. [PubMed.]
- Jahanbani J, Sandvik L, et al. (2009) Evaluation of oral mucosal lesions in 598 referred Iranian patients” Open Dent J. 3: 42-47. [PubMed.]
- Neville B, Damn D, Allen C, Bouquet J, et al. (2009) Philadelphia: Saunders Elsevier; Oral and Maxillofacial Pathology. Pp: 7-8. [Ref.]
- Güleç AT, Haberal M. (2010) Lip and oral mucosal lesions in 100 renal transplant recipients” J Am Acad Dermatol. 62: 96-101. [Ref.]
- Rossi M, Carpi A, et al. (2009) Skin blood flow-motion and microvascular reactivity investigation in hypercholesterolemic patients without clinically manifest arterial diseases” Physiol Res. 58: 39-47. [PubMed.]
- Image 1 Courtesy: pcds.org.uk. [Ref.]
- Image 2 Courtesy: Science direct. [Ref.]
- Image 3 Courtesy: Dentowsome.com. [Ref.]
- Image 4 Courtesy: Dentistry.uiowa.com. [Ref.]
- Image 5 Courtesy: Springer link. [Ref.]
- Image 6 Courtesy: Pathology out. [Ref.]