>Corresponding Author : Sara Chabbar SR
>Article Type : Research Article
>Volume : 5 | Issue : 1
>Received Date : 26 April, 2025
>Accepted Date : 06 May, 2025
>Published Date : 14 May, 2025
>DOI : https://doi.org/10.54289/JAAD2500102
>Citation : Chabbar SSR, Elabassi T, Faouji F, Mounir A and El Kettani C. (2025) Hemodynamic Variation After Mannitol Osmotherapy in Intracranial Surgery. J Anaesth Anesth Drug 5(1): doi https://doi.org/10.54289/JAAD2500102
>Copyright :© 2025 Chabbar SSR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access | Full Text
Surgical Intensive Care Unit, Central Operating Room, Ibn Rochd University Hospital Center, Casablanca, Morocco.
*Corresponding author: Chabbar Sara, Surgical Intensive Care Unit, Central Operating Room, Ibn Rochd University Hospital Center, Casablanca, Morocco.
Abstract
Mannitol osmotherapy is essential in neurosurgery to achieve adequate brain relaxation by increasing plasma osmolality, which draws water from the cerebral parenchyma into the vascular compartment. This improves cerebral perfusion and ensures optimal surgical conditions. However, mannitol administration induces significant hemodynamic variations.
Materials and Methods: This prospective observational study was conducted in the central operating room of Ibn Rochd University Hospital Center under the supervision of the Surgical Intensive Care Unit (Pavilion 17). Twenty three patients undergoing intracranial lesion excision in the neurosurgery suite between May and July 2023 were included. Adult patients with a Glasgow Coma Scale (GCS) score of 15/15 and no associated comorbidities (ASA 1) provided informed consent. After anesthesia induction, all patients received a fluid bolus of 20 cc/kg before mannitol administration. Continuous hemodynamic monitoring was employed to track responses.
Results: Initially, the transient increase in intravascular volume may elevate cardiac output (CO), which is generally well tolerated except in patients with cardiovascular comorbidities. This is followed by marked diuresis, transitioning from hypervolemia to hypovolemia, resulting in fluctuations in blood pressure (BP) and CO. Such shifts necessitate strict hemodynamic monitoring and precise fluid management to maintain stability.
The main clinical challenges involve fluid management and hemodynamic surveillance. The key principle is an individualized approach—especially for high risk cardiac patients—and the recommendation for continuous CO monitoring. Thorough preoperative assessment and interdisciplinary collaboration are essential to minimize risks. Proactive management of hemodynamic variations through appropriate fluid adjustments is critical to prevent complications. Future research should refine mannitol osmotherapy protocols and develop advanced hemodynamic monitoring technologies for more precise clinical interventions.
Conclusion: Despite its importance, mannitol osmotherapy requires rigorous monitoring to prevent complications and optimize clinical outcomes.
Abbreviations: GCS: Glasgow Coma Scale, CO: Cardiac Output, BP: Blood Pressure, ICP: Intracranial Pressure, ASA: American Society of Anesthesiologists, NIBP: Non Invasive Blood Pressure, TIVA: Total Intravenous Anesthesia, PIP: Peak Inspiratory Pressure, MAP: Mean Arterial Pressure, SVV: Stroke Volume Variation, CI: Cardiac Index
Introduction
Managing patients undergoing intracranial surgery presents unique and complex challenges. Among the various therapeutic strategies available, mannitol osmotherapy is widely used to reduce intracranial pressure (ICP) and improve surgical conditions.
Mannitol, an osmotic diuretic, creates an osmotic gradient upon administration that draws water from the cerebral parenchyma into the vascular compartment. This reduces cerebral edema and intracranial pressure.
However, mannitol administration is not without consequences and may induce significant hemodynamic variations. Initially, mannitol’s osmotic effect increases intravascular volume, which can lead to a transient rise in cardiac output. Subsequently, its powerful diuretic properties cause marked diuresis, reducing intravascular volume.
This transition from hypervolemia to hypovolemia can provoke abrupt fluctuations in blood pressure and cardiac output, necessitating close monitoring and proactive fluid management. Continuous hemodynamic monitoring is essential to detect and manage these shifts.
This study aims to examine the effects of mannitol osmotherapy on hemodynamic parameters in patients undergoing intracranial surgery. By analyzing the changes induced by mannitol and their impact on perioperative management, we seek to provide valuable data to practitioners, thereby improving care for neurosurgical patients.
We will first explore the principles of osmotherapy and the mechanisms of action of mannitol, along with a review of the physiological and pathophysiological concepts of intracranial hypertension, cardiac output, and hypovolemia. Next, we will examine the hemodynamic effects observed through analysis of our own clinical observations. Finally, we will discuss the implications of these results for clinical practice and offer recommendations for the optimal management of patients undergoing intracranial surgery.
Prospective Observational Study
Conducted in the Central Operating Room of Ibn Rochd University Hospital under the supervision of the Surgical Intensive Care Unit (Pavilion 17), Casablanca, Morocco.
Twenty three adult patients were enrolled between May and July 2023. All were scheduled for neurosurgical excision of an intracranial space occupying lesion and met the following inclusion criteria:
• Provided informed consent
• Glasgow Coma Scale (GCS) 15/15
• American Society of Anesthesiologists (ASA) physical status I
Exclusion Criteria
• Hypertension or diabetes with preexisting nephropathy
• Renal insufficiency
• Incomplete data
• Chronic diuretic, ACE inhibitor/ARB, or beta blocker therapy
• Children or pregnant women
• Emergency surgery cases
Perioperative Protocol
• After anesthesia induction, each patient received a standardized fluid bolus of 20 cc/kg.
• Mannitol was then infused at 0.5 g/kg over 30 minutes, commenced immediately following fluid resuscitation, during craniotomy.
• The dura mater was only opened once both hemodynamics were stabilized and adequate brain relaxation was confirmed by the surgeon’s manual assessment.
Hemodynamic Monitoring
• Non invasive: non invasive blood pressure (NIBP), SpO₂, ECG, urinary catheter, transthoracic echocardiography
• Invasive: right radial arterial catheter with a Flotrac™ sensor connected to the Vigileo™ system (Figure 1).
Data were collected continuously before and after induction and fluid resuscitation for all patients.
Ventilatory and Anesthetic Protocol
• Timing of Measurements:
o Pre osmotherapy: 10 minutes after fluid resuscitation
o Post osmotherapy: 20 minutes after completion of mannitol infusion
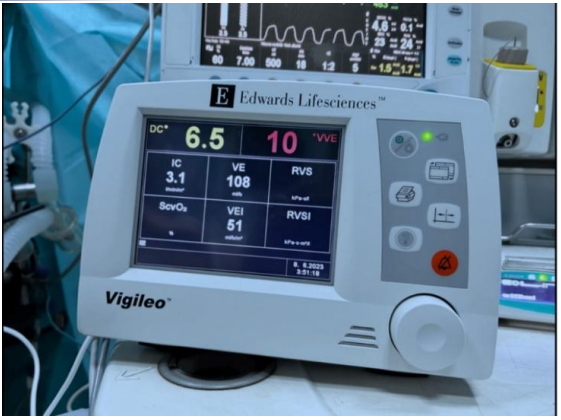
Figure 1. Invasive monitoring included a radial arterial catheter placed in the right radial artery, with a Flotrac™ sensor connected to the Vigileo™ system.
• Anesthesia:
o Total intravenous anesthesia (TIVA) with propofol
o No inhaled halogenated agents (to avoid increased cerebral blood flow, elevated ICP, and bleeding)
• Initial Ventilator Settings:
o Tidal Volume (Vt): 6–8 mL/kg of ideal body weight
o Respiratory Rate: 12–15 breaths/min
o I:E Ratio: 1 : 2
o FiO₂: 50–100 % (to maintain satisfactory SpO₂)
• Targeted Ventilatory Goals
o Peak Inspiratory Pressure (PIP): < 30 cmH₂O (minimize barotrauma)
o Plateau Pressure: < 30 cmH₂O (prevent ventilator induced lung injury)
o End Tidal CO₂ (EtCO₂): 33–35 mmHg (correlates with PaCO₂ in healthy lungs)
All parameters were continuously adjusted during surgery to meet individual patient requirements and maintain optimal gas exchange and cerebral protection.
Results
The study sample exhibited a male predominance, although this bears no analytical relevance in our work. Nevertheless, investigating sex related variations could be an interesting avenue for future research.
All patients (100 %) in our cohort were admitted for tumor excision, and all lesions were located supratentorially.
The clinical parameters analyzed in our study were as follows (Figure 2):
• Heart rate
• Blood pressure: systolic and diastolic
• Mean arterial pressure (MAP)
• ΔP (pulse pressure difference)
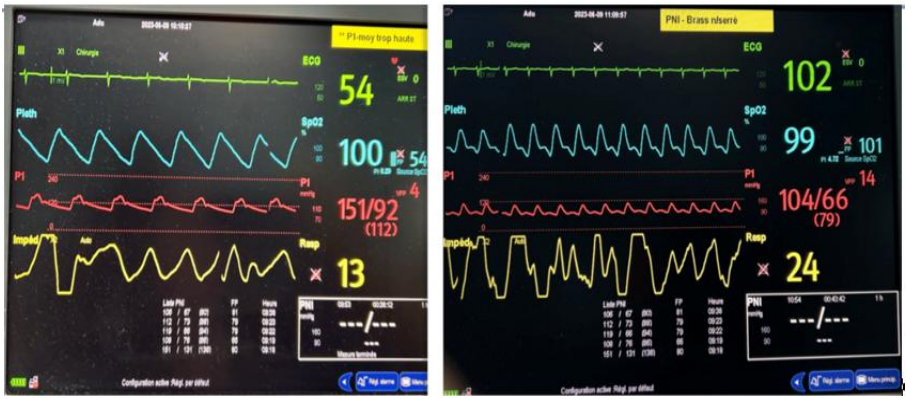
Figure 2. Hemodynamic parameters of the same patient before and after osmotherapy
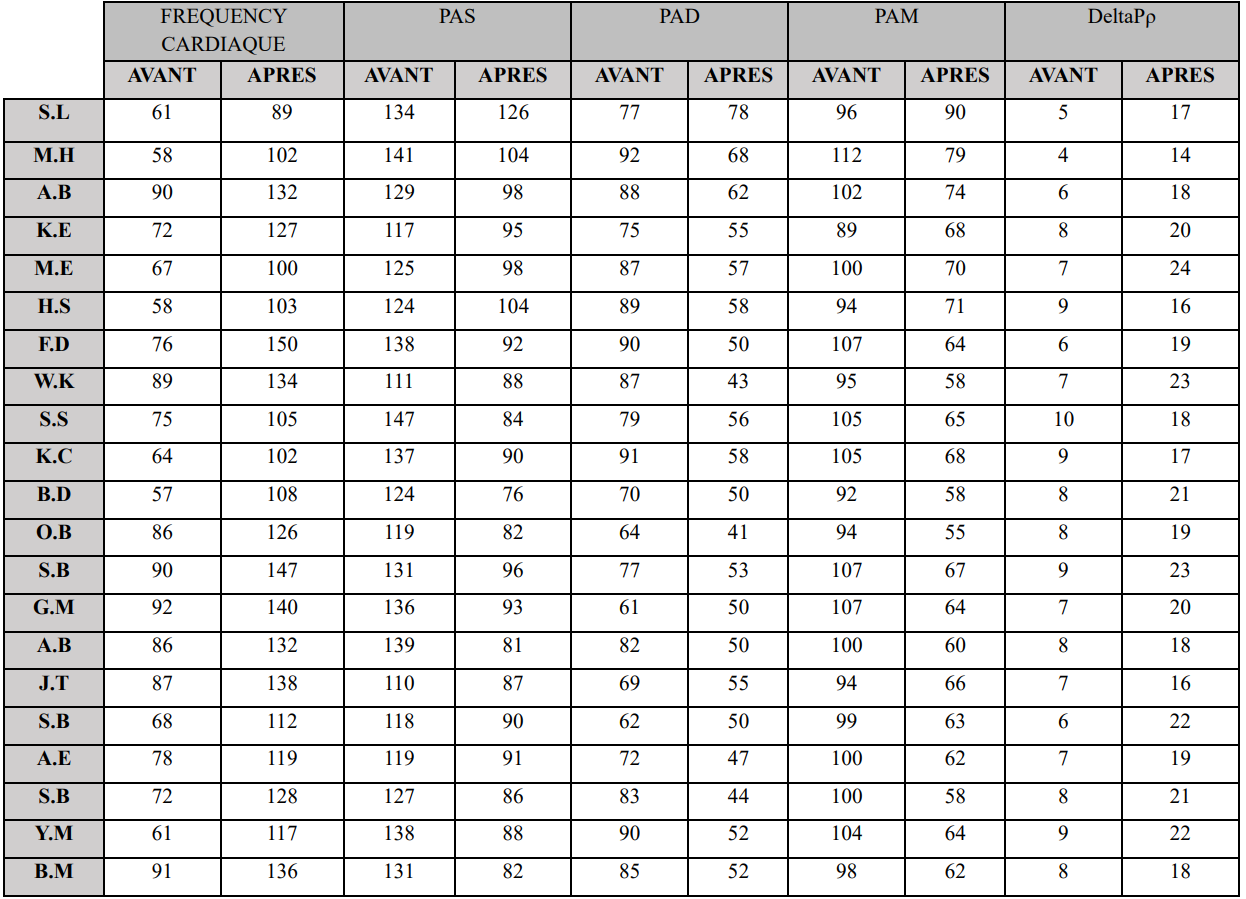
Table 1. Variations in hemodynamic parameters before and after osmotherapy.
All measured parameters showed a significant decrease after the mannitol infusion, except for heart rate.
• Heart Rate: Increased by an average of 41% post infusion, from a baseline of 75 ± 17 bpm. Values as high as 147 bpm were recorded on multiple occasions.
• Blood Pressure: On average, systolic and diastolic pressures fell by 33% and 23%, respectively, resulting in a 31% drop in mean arterial pressure (Table 1).
Invasive Hemodynamic Data (Vigileo™):
• Stroke Volume (SV)
• Stroke Volume Variation (SVV)
• Cardiac Output (CO)
• Cardiac Index (CI)
All of these parameters converged to show a decrease in stroke volume and, consequently, cardiac output and cardiac index.
• SVV Change: The average increase in SVV following osmotherapy was 16.86%, exceeding the normal threshold of 12% (Figure 3).
Cardiac Output (CO) and Cardiac Index (CI) likewise experienced a significant decline, mirroring the reduction in Stroke Volume (SV) despite the concomitant tachycardia noted earlier.
Cardiac Output and Stroke Volume Changes
• Cardiac Output (CO): Decreased by an average of 43.83% following mannitol infusion.
• Stroke Volume (SV): Fell by an average of 44.15% post infusion.
Notably, a paradoxical increase in both CO and SV was observed during the initial moments of the infusion; this phenomenon will be discussed in more detail later.
Cardiac Index (CI): Exhibited the same downward trend as CO (Figure 4). Overall, these parameters declined by an average of 38.96%.
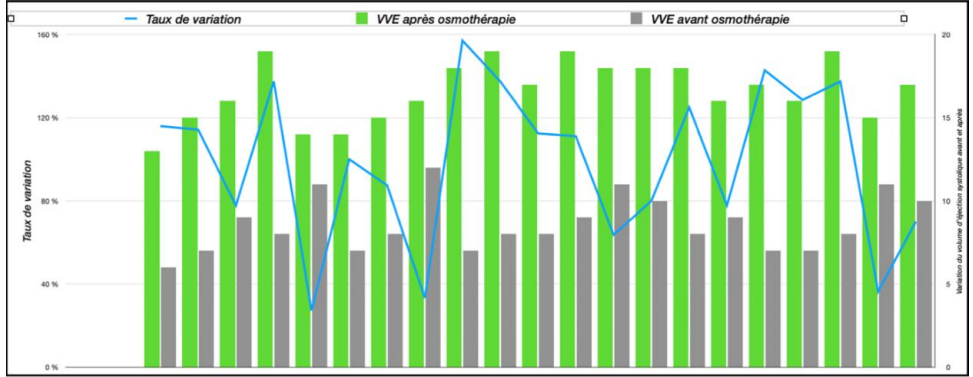
Figure 3. Changes in LVESV (Left Ventricular End-Systolic Volume) before and after osmotherapy.
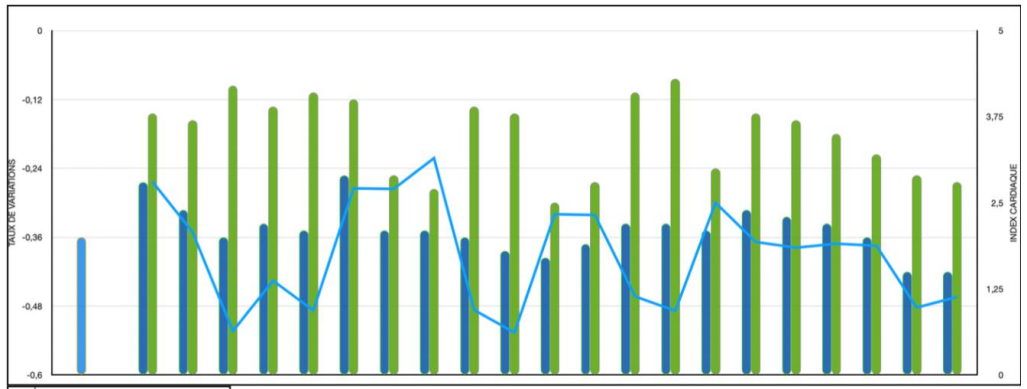
Figure 4. Cardiac index variations before and after osmotherapy.
Echocardiographic Parameter Changes
The echocardiographic parameters analyzed were:
• Respiratory variability of the inferior vena cava diameter (ΔIVC)
• Subaortic velocity–time integral (VTI)
These markers are considered echocardiographic correlates of the previously described clinical and hemodynamic parameters (Table 2).
We observed a significant increase in respiratory variability of the IVC diameter following mannitol infusion. This reduction averaged 37.76%.
Regarding subaortic VTI (velocity-time integral), we noted a parallel decrease corresponding to the reduction in cardiac output, with an average decline of 36.3%.
Discussion
Given the effects of osmotherapy on cerebral osmoregulation and, subsequently, on brain relaxation, this therapeutic approach remains a cornerstone in the neurosurgical operating room [1]. However, it is not without concomitant side effects, as observed in our study [2].
As previously highlighted, mannitol—through its osmotic properties—induces an effective osmotic diuresis. When uncontrolled, this leads to significant hypovolemia in patients. This aligns precisely with our findings.
Such hypovolemia reduces venous return, resulting in decreased stroke volume and cardiac output.
This reduction persists despite compensatory tachycardia mediated by sympathetic activation. In certain patients—particularly those with coronary artery disease or ischemic heart disease—this hemodynamic profile may prove deleterious.
The combination of a pronounced decrease in stroke volume (SV) with tachycardia (reducing diastolic filling time while increasing myocardial oxygen demand) could have devastating consequences for this patient population [2]. These findings underscore that hypovolemia carries significant clinical implications, both pathophysiologically and quantitatively. A near 50% reduction in cardiac output with comparable decreases in stroke volume constitutes a hemodynamic crisis requiring immediate intervention.
At the tissue level, compensatory mechanisms activate to counteract oligemia. While initially effective, these may become overwhelmed, potentially progressing to end-organ ischemia. Notably, clinicians should remain vigilant for a paradoxical early-phase response: transient cardiac output elevation occurring within minutes of infusion initiation.
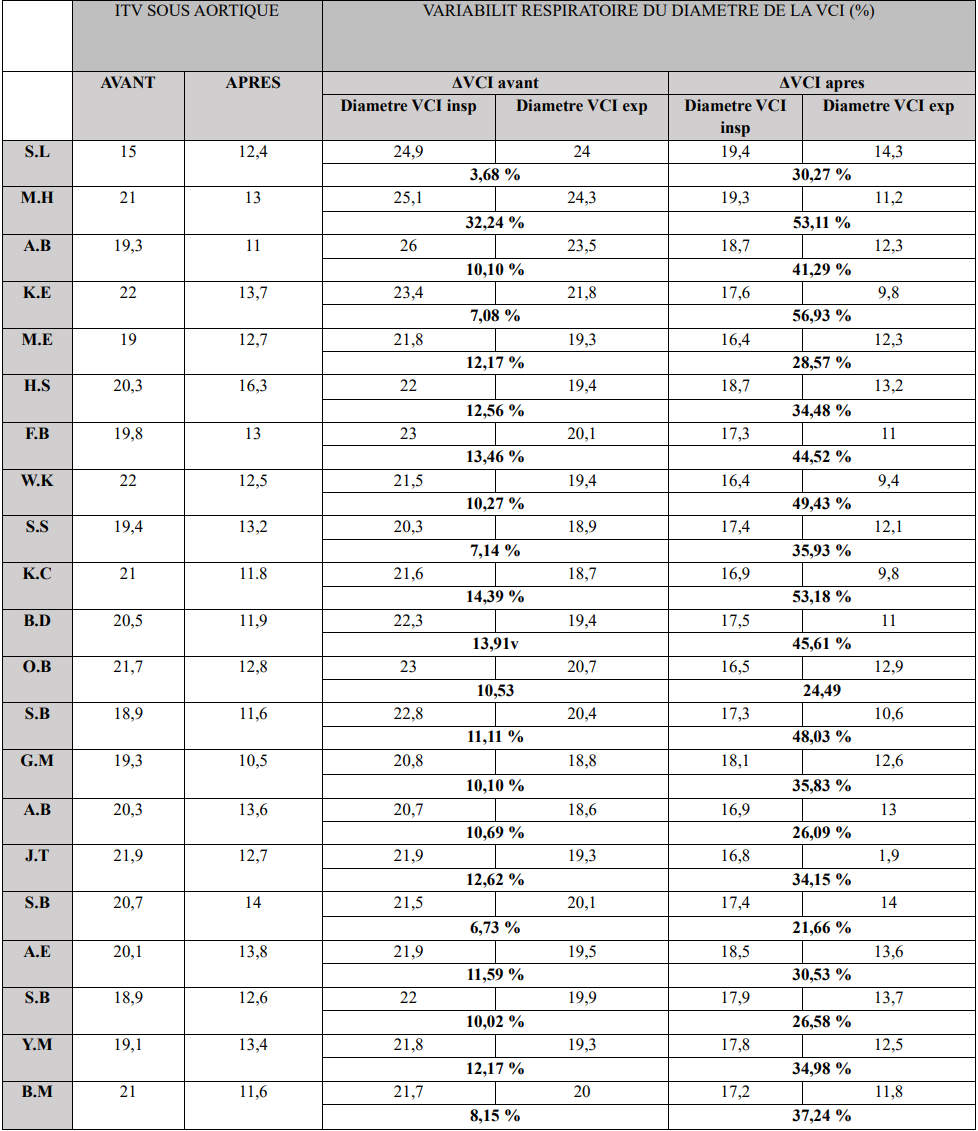
Table 2. Echocardiographic Parameter Variations.
This phenomenon stems from mannitol's osmotic properties, which induce fluid shifts that transiently expand intravascular volume. The resultant preload increase temporarily augments cardiac output (CO). However, in patients with cardiac comorbidities, this apparent hemodynamic "improvement" carries substantial risk—particularly the potential to precipitate acute decompensated heart failure.
In healthy individuals, this transient volume expansion is generally well tolerated, as demonstrated by several studies [3,4]. Some authors have even suggested that this brief hemodynamic surge may enhance cerebral perfusion, albeit transiently [5].
Subsequently, the diuretic effect predominates. Osmotic diuresis reduces intravascular volume, ultimately precipitating hypovolemia—the key driver of cardiac output decline, as previously observed. This creates a state of preload dependence.
While compensatory mechanisms (activated via volume and baroreceptor stimulation) may initially mitigate mild hypovolemia, they prove inadequate in severe cases, as evidenced by VigileoTM monitoring data. Our study consistently demonstrated classic low-cardiac-output markers following osmotic diuresis.
Notably, the timing of this low-output state varied significantly among patients, sometimes manifesting only after infusion cessation. This variability precludes standardized "blind" fluid management and underscores the necessity for continuous—potentially invasive—hemodynamic monitoring.
Echocardiographic assessment served a singular purpose: to validate and supplement quantitative hemodynamic data. The temporal dimension remains critical—prolonged low-output states exacerbate end-organ damage through sustained hypoperfusion [6].
However, routine echocardiographic monitoring presents practical challenges, limiting its feasibility for everyday clinical use despite its diagnostic value.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations ethics approval: The study was conducted in accordance with the ethical principales of the declaration of helsinki
Ethics Committee: (File No. 9/2025).
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest
Conclusion
Mannitol osmotherapy remains a cornerstone intervention in neurosurgery for managing intracranial hypertension. In the operating room during scheduled procedures, it is indispensable for achieving optimal brain relaxation, thereby improving surgical conditions.
This thesis has provided an in-depth exploration of the mechanisms, effects, and clinical implications of mannitol-induced hemodynamic variations. Mannitol primarily acts by increasing plasma osmolality, creating an osmotic gradient that draws fluid from cerebral parenchyma into the vascular compartment.
Our findings highlight the need for further research to refine mannitol osmotherapy protocols, particularly in light of the observed hemodynamic fluctuations. Comparative studies evaluating different dosing regimens and infusion rates could help identify the safest and most effective strategies.
Additionally, advancements in hemodynamic monitoring technologies—such as non-invasive, real-time systems—may enhance the management of mannitol-induced changes, enabling more precise clinical decision-making.
In summary, while mannitol osmotherapy is a critical tool in neurosurgical intracranial hypertension management, its hemodynamic effects demand rigorous monitoring and proactive fluid management to mitigate complications. This study underscores the importance of thorough preoperative assessment, continuous intraoperative surveillance, and individualized fluid strategies to optimize outcomes. Future research and technological innovations will be pivotal in refining the safety and efficacy of this essential therapy.
References:
- Mangat HS., Wu X., Gerber LM., et al. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. British Journal of Anaesthesia. 2015;122(1):202‒10. [PubMed.]
- Israel RS., Marx JA., Moore EE., Lowenstein SR. Hemodynamic effect of mannitol in a canine model of concomitant increased intracranial pressure and hemorrhagic shock. Annals of Emergency Medicine.1988;17(6):560‒6. [PubMed.]
- Myburgh JA. Mannitol A review of its clinical uses. Continuing Education in Anaesthesia, Critical Care & Pain. 2012;12(3):82‒85. [Ref.]
- Cloyd JC., Snyder BD., Cleeremans B., et al. Mannitol pharmacokinetics and serum osmolality in dogs and humans.Journal of Pharmacology and Experimental Therapeutics.1986;236(2):301‒306. [PubMed.]
- Beard DA., Feigl EO. Understanding Guyton’s venous return curves. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301(3):H629‒H633. [PubMed.]
- Bendjelid K. Non-invasive cardiac output monitoring: A contemporary review. Revue Médicale Suisse. 2018;14(623):2125‒2130. [Ref.]