>Corresponding Author : Benaddi L
>Article Type : Research Article
>Volume : 5 | Issue : 1
>Received Date : 21 April, 2025
>Accepted Date : 03 May, 2025
>Published Date : 07 May, 2025
>DOI : https://doi.org/10.54289/JAAD2500101
>Citation : Benaddi L, Chabbar S, Boujmai L, Berrada L, Zerhouni A, et al. (2025) Perioperative Management of Antiplatelet Agents by Anesthesiologists and Cardiologists. J Anaesth Anesth Drug 5(1): doi https://doi.org/10.54289/JAAD2500101
>Copyright :© 2025 Benaddi L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access | Full Text
Surgical Intensive Care Unit, University Hospital Ibn Rochd of Casablanca
*Corresponding author: Loubna Benaddi, Surgical Intensive Care Unit, University Hospital Ibn Rochd of Casablanca
Abstract
Antiplatelet agents (APAs) are essential in the management of atheromatous disease. The number of patients on long-term antiplatelet therapy who require scheduled invasive procedures is increasing each year. Managing antiplatelet therapy in the perioperative period remains a significant challenge for both cardiologists and anesthesiologists. Achieving a balance between the hemorrhagic risk associated with surgery and the thrombotic risk from modifying antiplatelet therapy is crucial.
Our study compared the perioperative management of antiplatelet agents among 50 anesthesiology and intensive care residents and 43 cardiology residents at our university hospital, referencing European and American guidelines. The results indicate that cardiology residents tend to prioritize the thrombotic risk, minimizing the discontinuation period of antiplatelet agents. In contrast, anesthesiology residents are more cautious regarding hemorrhagic risk, especially in the management of anti-P2Y12 monotherapy and dual antiplatelet therapy, often opting for longer discontinuation periods.
In line with our findings and the literature, both specialties strive to balance thrombotic and hemorrhagic risks, though cardiologists may focus more on thrombotic complications, while anesthesiologists emphasize bleeding risk. The therapeutic approach is inherently multidisciplinary and should be individualized based on patient-specific and procedural risks, as endorsed by current guidelines.
Abbreviations: APAS: Antiplatelet Agents, PCI: Percutaneous Coronary Intervention, ACS: Acute Coronary Syndrome, DAPT: Dual Antiplatelet Therapy, ADP: Adenosine Diphosphate, ROTEM: Rotational Thromboelastometry, HAS: Haute Autorité De Santé, ESC: European Society of Cardiology, NSAIDS: Nonsteroidal Anti-Inflammatory Drugs
Introduction
Antiplatelet agents (APAs) are crucial in managing atheromatous disease, primarily to prevent arterial thrombosis and reduce the risk of recurrent acute atherothrombotic events. Surgery is a common reason for premature discontinuation of antiplatelet therapy, which significantly increases mortality and major adverse cardiac events, notably stent thrombosis [1]. The management of antiplatelet therapy during the perioperative period poses a significant challenge in clinical practice for both prescribing physicians and anesthesiologists. This challenge arises from the need to balance the risk of bleeding associated with the continuation of antiplatelet agents against the risk of thrombosis if these medications are discontinued [1]. Clinical practice guidelines offer limited guidance on managing antiplatelet therapy during the perioperative phase for patients undergoing non-deferrable surgeries or those with high hemorrhagic risk [2].
The objective of your study is to evaluate the management of antiplatelet agents (APAs) from the perspectives of anesthesiologists and cardiologists at our university hospital, identify commonalities and differences, and compare these practices to international guidelines.
Materials and Methods
This study is a cross-sectional descriptive study conducted among residents in anesthesiology and cardiology at the Ibn Rochd University Hospital in Casablanca over a period of four months, from September 2023 to December 2023. Senior practitioners from each specialty were excluded from the study. Data collection was performed using an anonymous electronic questionnaire.
Initially, we aimed to determine how frequently these practitioners encounter patients on mono or dual antiplatelet therapy in a surgical context, whether in emergency situations or scheduled procedures. Subsequently, we evaluated their knowledge, documented their practices, and compared them regarding the perioperative management of these patients, focusing on the interruption or continuation of treatment, the duration of discontinuation, the timing of resumption, and the precautions taken.
Results
Distribution of our population
93 residents responded to the questionnaire, of which 50 (54%) are undergoing training in anesthesiology and intensive care.
Their distribution according to the training years is shown in the chart below (Figure 1).
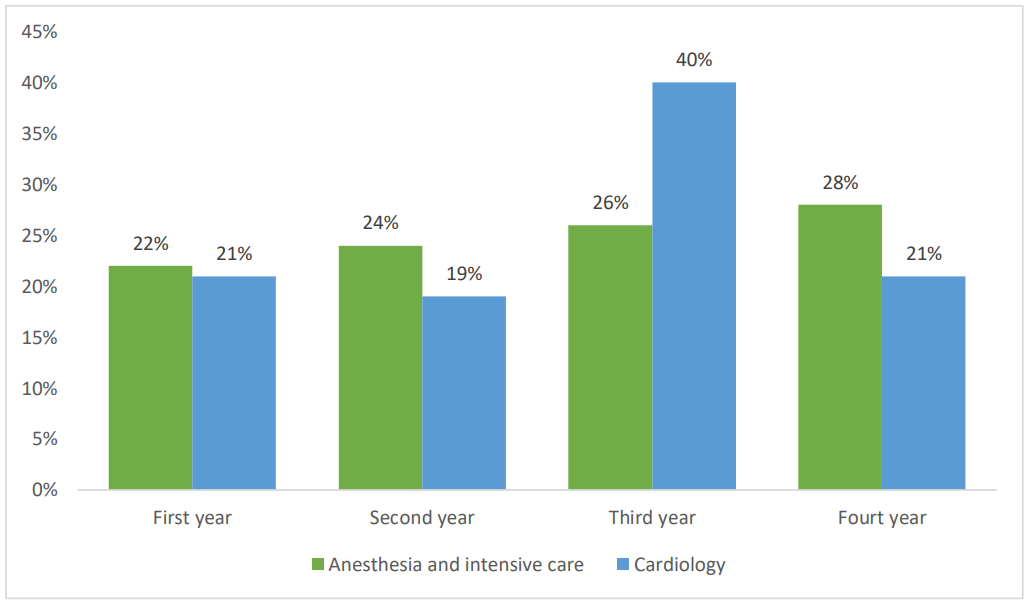
Figure 1. Chart showing the distribution of the residents in the study according to years of training.
Frequency of managing patients on antiplatelet agents in the perioperative period
The survey results show that all residents have encountered patients on antiplatelet agents.
Here are the details on the frequency of consultation:
- Preoperative: 69% of residents managed these patients during pre-anesthetic consultations.
Among them, 29 residents were in cardiology.
- Peri and postoperative: 31% managed these patients during the peri and postoperative periods, including 15 residents in anesthesiology and intensive care.
73% of the population often manage patients on dual antiplatelet therapy. 26% encountered this situation in the context of emergency surgeries.
Our survey also revealed the following practices among participants:
- 88% of anesthesiologists and intensivists participating in the study refer patients with coronary stents to cardiologists for preoperative cardiac evaluation, risk stratification, and management of antiplatelet agents.
54% of anesthesiologists and intensivists request troponin levels preoperatively for these patients. Complete blood count and coagulation studies are systematically requested by anesthesiologists as part of pre-anesthetic consultations.
- Conversely, 74% of cardiologists do not request any preoperative biological tests.
Estimation of hemorrhagic risk
The surgeries identified as high hemorrhagic risk by anesthesiology and intensive care residents are as shown below (Figure 2):
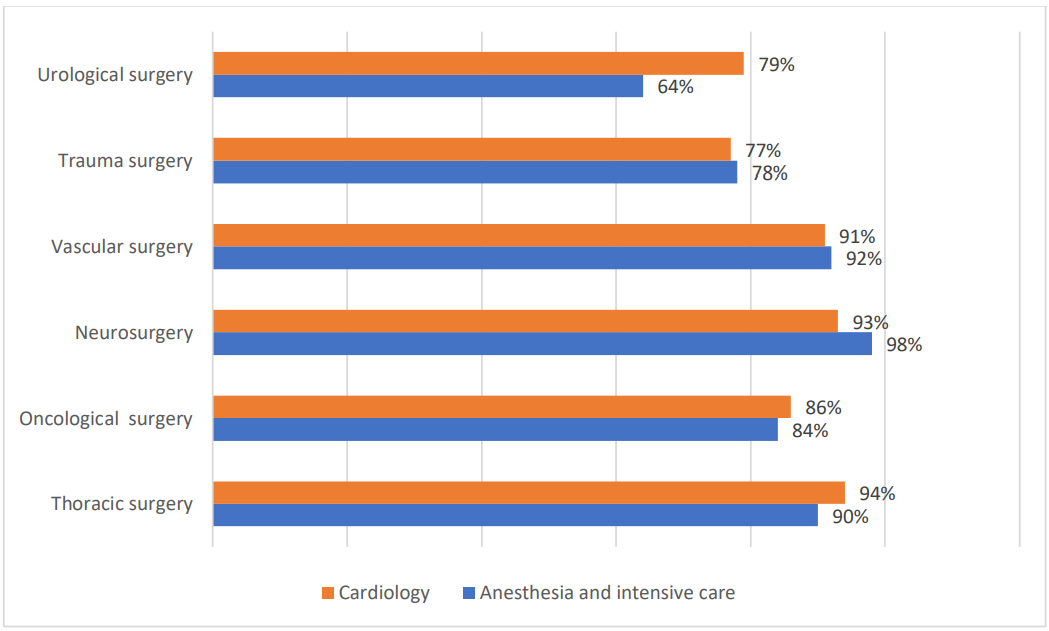
Figure 2. Chart showing the high bleeding risk surgeries according to anesthesiologists and cardiologists.
The majority of residents from both specialties agree that the following procedures are associated with low bleeding risk: anterior segment eye surgery, dental surgery, and endoscopic procedures. They also identify surgeries with intermediate bleeding risk as:
visceral surgery, ent (ear, nose, and throat) surgery, maxillofacial surgery, posterior segment eye surgery.
Estimation of Thrombotic Risk
98% of anesthesiology residents consider thrombotic risk to be high in cases of percutaneous coronary intervention (PCI) performed less than one month prior. While 77% perceive a high thrombotic risk for acute coronary syndrome (ACS) occurring within the past three months, only 31% consider the risk to remain high when ACS occurred between three and six months ago.
Concerning cardiology residents, all of them agree that thrombotic risk is high for PCI performed less than one month prior. They also consider thrombotic risk to be elevated for ACS within the past six months.
The consensus among cardiology residents highlights their heightened awareness of prolonged thrombotic risks, particularly in cases involving recent coronary interventions or acute coronary events.
Practice for managing hemorrhagic and thrombotic risks:
Management of monotherapy
The study revealed the following practices regarding aspirin monotherapy management:
- Aspirin taken as self-medication: 70% of anesthesiologists and intensivists participating in the study stop aspirin taken as selfmedication without indication, without consulting a cardiologist.
All cardiologists stop aspirin taken as self-medication when there is no indication.
Aspirin for primary prevention: 100% of residents, of both specialties, discontinue aspirin prescribed for primary prevention, regardless of the hemorrhagic risk of the surgery.
Aspirin for secondary prevention: 98% of anesthesiologists and intensivists continue aspirin monotherapy for surgeries with low or intermediate hemorrhagic risk, while all of cardiologists continue it in these surgeries. All of residents discontinue it for surgeries with high hemorrhagic risk.
The study findings outline the practices regarding clopidogrel monotherapy for secondary prevention:
- High hemorrhagic risk surgeries: All residents, regardless of specialty, discontinue clopidogrel monotherapy for surgeries with high hemorrhagic risk, without switching to aspirin.
- Low hemorrhagic risk surgeries: Among cardiologists, 100% continue clopidogrel monotherapy for surgeries with low hemorrhagic risk and 73% discontinue it and switch to aspirin for surgeries with low hemorrhagic risk. Concerning anesthesiologists and intensivists, 79% discontinue clopidogrel and switch to aspirin for surgeries with low or intermediate hemorrhagic risk.
Management of dual antiplatelet therapy
The study findings outline the practices for managing dual antiplatelet therapy (DAPT) in perioperative settings according to thrombotic risk.
- High Thrombotic Risk: All residents propose deferring surgery when possible.
If surgery cannot be postponed, 100% of cardiology and anesthesiology residents discontinue DAPT for surgeries with high hemorrhagic risk.
For surgeries with low hemorrhagic risk, 88% of cardiologists continue DAPT while only 67% of anesthesiologists adopt the same approach.
For intermediate hemorrhagic risk, all residents discontinue the P2Y12 inhibitor and continue aspirin.
- Low Thrombotic Risk: For surgeries with high hemorrhagic risk, all residents discontinue DAPT.
For intermediate hemorrhagic risk, all residents stop the P2Y12 inhibitor and continue aspirin. Whereas for low hemorrhagic risk, 43% of anesthesiologists and 74% of cardiologists continue DAPT, which is lower compared to scenarios involving high thrombotic risk.
Only two anesthesiology residents suggested using cangrelor and anti-GPIIb-IIIa as a bridging alternative in cases of surgeries with very high thrombotic and hemorrhagic risks.
Duration of antiplatelet agent discontinuation
All residents agreed on the duration for discontinuing antiplatelet agents:
- Aspirin: 3 days
- Clopidogrel and Ticagrelor: 5 days - Prasugrel: 7 days.
For neurosurgery, particularly intracranial procedures, 92% of anesthesiologists and intensivists (46 out of 50 residents) recommended extending the antiplatelet discontinuation period. In contrast, only 59% of cardiologists (25 out of 43 residents) agreed with this recommendation.
Prevention of hemorrhage in emergency surgery
Regarding emergency surgery, 86% of anesthesiologists recommend platelet transfusion when the patient is on aspirin alone and all of them resort to platelet transfusion when other antiplatelet agents (e.g., clopidogrel, ticagrelor) are involved. Conversely, all cardiologists recommend platelet transfusion in both scenarios (Figure 3).
Red blood cell transfusion is only considered in cases of associated hemorrhage requiring transfusion, a practice shared by both specialties.
Furthemore, 74% of anesthesiologists and only 38% of cardiologists support the use of tranexamic acid to manage bleeding risks. ROTEM is considered advantageous by 77% of anesthesiologists for guiding hemostatic management in these scenarios whereas 39% of cardiologists agree with this assessment (Figure 3).
Resumption of treatment
All intensivists resume aspirin therapy 6 hours after surgery if there is no bleeding and no coagulation disorders. For P2Y12 inhibitors, they are resumed in combination with aspirin only 24 hours after surgery, provided there is no risk of hemorrhage or bleeding.
For cardiologists, 94% resume aspirin 24 hours after surgery, while the remaining 6% resume it 12 hours postoperatively, provided there is no bleeding. All of them resume P2Y12 inhibitors in combination with aspirin 48 hours after surgery, assuming there is no bleeding or hemorrhagic risk.
Discussion
Patients undergoing long-term antiplatelet therapy frequently require scheduled invasive procedures each year. These procedures need specific management of one or more antiplatelet agents (APAs).
The four main oral APAs target two distinct pharmacological pathways:
• Aspirin inhibits the cyclooxygenase-1 enzyme, thereby suppressing thromboxane A2 synthesis.
• Clopidogrel, prasugrel, and ticagrelor inhibit the adenosine diphosphate (ADP) pathway via the platelet P2Y12 receptor.
It is essential to evaluate the hemorrhagic risk associated with the procedure and its feasibility under APAs therapy, as well as the thrombotic risk linked to any potential modification of the treatment [1].
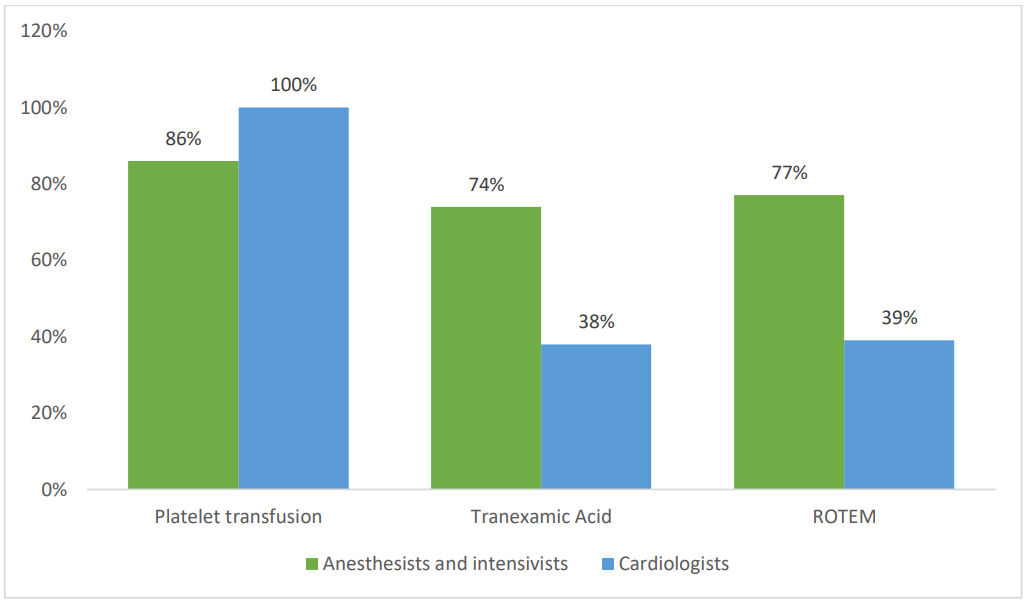
Figure 3. The preventive measures taken by cardiologists and anesthesiologists regarding the hemorrhagic risk in emergency surgeries under antiplatelet agents.
Hemorrhagic risk associated with continuing antiplatelet agents
The hemorrhagic risk induced by antiplatelet agents varies depending on the invasive procedure and includes not only the volume of bleeding and the need for transfusion but also complications such as hematoma formation in functional surgeries or the requirement for surgical revision. The key question is identifying situations where the increased hemorrhagic risk is unacceptable, requiring peri-procedural modifications of antiplatelet agents prescription.
While aspirin is perceived to induce a lower hemorrhagic risk compared to clopidogrel, this distinction is not definitively established. However, clopidogrel generally poses less hemorrhagic risk than newer P2Y12 inhibitors, such as ticagrelor and prasugrel. Dual therapy (aspirin + P2Y12 inhibitor) presents a higher hemorrhagic risk than monotherapy, typically involving aspirin alone [2].
Procedures classified as high hemorrhagic risk are considered unfeasible under AAP therapy, even with aspirin monotherapy. These procedures are rare and include certain urological interventions, many intracranial neurosurgeries, trauma surgeries involving extensive damage or large dissections, and specific hepatic, thoracic, or vascular surgeries. This assessment aligns with findings from our study [3].
Intermediate-risk procedures encompass the majority of invasive interventions, including visceral and ENT (ear, nose, and throat) surgeries.
Procedures with low hemorrhagic risk include, for example, cataract surgery, certain dental surgeries, certain urological procedures such as urethrocystoscopy, some bronchoscopies, and certain digestive endoscopies: all diagnostic endoscopies with or without biopsies, endoscopic retrograde cholangiopancreatography without sphincterotomy, and colonic polypectomies less than 1 cm [3].
When there is no consensus or reference framework for categorizing an invasive procedure into one of these risk categories, it is proposed that a reference team (operator, anesthesiologist, cardiologist, pulmonologist, vascular surgeon, etc.) within the healthcare facility define a management approach on a case-by-case basis, either for a specific patient profile or procedure type. These decisions are documented in the patient's file or in the facility's procedures [1].
Duration of antiplatelet agents interruption and bridging
The optimal duration for interrupting an APA before an invasive procedure is the shortest time that reduces the increased hemorrhagic risk associated with APA therapy
No hematostatic safety threshold has been established to guarantee the absence of periprocedural hemorrhagic risk due to residual APA effects, regardless of the functional platelet test used in the laboratory or at the patient's bedside. Consequently, only a minority of cardiologists in the study viewed rotational thromboelastometry (ROTEM) as a beneficial tool [4].
Aspirin irreversibly inhibits thromboxane A2 synthesis. The time required for complete recovery of thromboxane A2 synthesis capacity is equivalent to the lifespan of circulating platelets, which is normally ten days. However, this recovery does not need to be complete for the full correction of platelet functions dependent on this synthesis or for sufficient hemostatic competence to handle the invasive procedure [3].
There is interindividual variability in correcting platelet functions, and not all subjects achieve complete correction within four days. Furthermore, a faster recovery of platelet functions inhibited by aspirin can occur in some patients, such as those with accelerated platelet turnover, like diabetics or individuals with thrombocytosis due to a myeloproliferative neoplasm [4].
The HAS recommends discontinuing aspirin with a three-day interval of non-use. For procedures with high hemorrhagic risk, the goal is to completely correct the platelet functions inhibited by antiplatelet agents, and this objective must be achieved for all patients undergoing these procedures. Therefore, it is proposed to perform invasive procedures with high hemorrhagic risk, such as intracranial neurosurgery, after five days of aspirin discontinuationv [5].
The recommendations from the Haute Autorité de Santé (HAS) and the European Society of Cardiology (ESC) suggest discontinuing clopidogrel and ticagrelor with a five-day interval before invasive procedures, and prasugrel with a seven-day interval. The ESC/ESA guidelines are comparable in this regard [1,4].
These recommendations also apply to spinal anesthesia, the placement and removal of epidural catheters, and deep nerve blocks, requiring the same durations of discontinuation. For intracranial neurosurgery, it is proposed that the last dose of clopidogrel and ticagrelor be taken seven days prior to surgery, and for prasugrel, nine days before the procedure [3].
The literature supports our study's findings regarding the duration of antiplatelet agent discontinuation. Although cardiologists were not numerous in suggesting a longer discontinuation period for intracranial surgery, the recommended durations align with established guidelines.
Neither the literature nor our study identified a need for bridging therapy with heparins (unfractionated heparin or low molecular weight heparin) or nonsteroidal anti-inflammatory drugs (NSAIDs) in the perioperative management of antiplatelet agents [1].
Management of antiplatelet agents (APAs) based on their indication
Cardiovascular prevention:
According to the literature, aspirin should be discontinued preoperatively when prescribed for primary prevention. This recommendation is unanimously applied by cardiology and anesthesiology residents.
Moreover, Aspirin is not recommended to be discontinued preoperatively when prescribed for secondary cardiovascular prevention (e.g., post-ischemic stroke, coronary artery disease, peripheral artery disease), except in cases of high hemorrhagic risk procedures [3].
When anti-P2Y12 agents are used in monotherapy, clopidogrel is generally preferred. For surgeries with intermediate hemorrhagic risk, clopidogrel should be replaced with aspirin. This substitution should occur more than five days before surgery to allow complete correction of platelet inhibition induced by the anti-P2Y12 agent, [6].
Anesthesiology residents in our study adopt a more cautious approach to anti-P2Y12 monotherapy, discontinuing it and replacing it with aspirin for both intermediate and low hemorrhagic risk procedures.
Management of dual antiplatelet therapy (DAPT in patients with coronary stents
Patients with coronary stents often undergo non-cardiac surgeries, with 4 to 15% requiring such interventions within a year after stent placement [5]. Managing these patients while they are on dual antiplatelet therapy (DAPT) involves several considerations:
- Increased periprocedural hemorrhagic risk: continuing DAPT during invasive procedures increases the risk of bleeding, particularly when both aspirin and a P2Y12 inhibitor are used.
- Risk of stent thrombosis: stopping DAPT, especially in the early period after stent placement, significantly increases the risk of stent thrombosis, which can be catastrophic.
- The consequences of delaying the procedure.
The periprocedural period is inherently risky for ischemic events due to the pro-inflammatory and pro-coagulant state induced by surgery. This environment can favor coronary thrombosis both at the stented segments and in the native coronary circulation. Therefore, the decision to proceed with a planned invasive procedure after coronary stenting should be made with careful consideration of the risk-benefit ratio, especially for high-risk procedures like tumor surgery or vascular surgery for an abdominal aortic aneurysm [6,7].
To reduce ischemic risk, as well as hemorrhagic and transfusion risks associated with continuing antiplatelet agents (APAs), it is recommended to delay invasive procedures whenever possible until the end of the recommended duration of dual antiplatelet therapy (DAPT). For patients on DAPT following an acute coronary syndrome (ACS) or stent placement with high thrombotic risk characteristics, it is proposed to postpone non-cardiac surgery beyond the sixth month after stent placement [6,7].
When delaying surgery until the end of DAPT is not feasible, the P2Y12 inhibitors should be discontinued according to previously mentioned stoppage durations, while aspirin is continued unless there is a high risk of bleeding. For surgeries with low hemorrhagic risk, DAPT can be maintained [1,7].
According to the findings of our study, the majority of cardiology residents consider the thrombotic risk and continue DAPT when the hemorrhagic risk is low. In contrast, anesthesiology residents tend to stop the P2Y12 inhibitor and continue aspirin alone.
For patients at very high risk of stent thrombosis, particularly those requiring discontinuation of DAPT within the first month, bridging with reversible parenteral antiplatelet agents has been considered. However, the use of rapidly reversible anti-GPIIb-IIIa agents (eptifibatide or tirofiban) as bridging therapy poses a potential increased risk of bleeding without established benefits in reducing persistent stent thrombosis risk [7,8].
The use of parenteral antiplatelet agents like cangrelor, a reversible P2Y12 inhibitor, offers an alternative in the perioperative setting due to its established antithrombotic effect and faster reversibility compared to anti-GPIIb-IIIa agents. However, none of these parenteral antiplatelet agents have a marketing authorization for this specific indication [1].
Postoperative resumption of antiplatelet agents
For procedures with intermediate or high hemorrhagic risk, it is recommended that the preoperative management of APAs and their postoperative resumption be discussed with the patient's cardiologist or a consulting cardiologist. This decision should be documented in the patient's medical record.
Aspirin should be resumed as early as possible after the invasive procedure, ideally on the same day, depending on the risk of postoperative bleeding [8].
If P2Y12 inhibitors were discontinued before surgery, they should be restarted promptly, ideally within 24 to 72 hours post-surgery, to mitigate the increased thrombotic risk. The same P2Y12 inhibitor used preoperatively should be resumed [1,8].
Cardiologists often favor shorter interruptions, while anesthesiologists may advocate longer pauses in high bleeding risk cases as shown by our findings.
Conclusion
The perioperative management of antiplatelet agents (APAs) requires a thorough evaluation of both thrombotic and hemorrhagic risks, with specific recommendations tailored to the indications and types of procedures to reduce perioperative morbidity and mortality. The approach varies depending on whether the procedure is scheduled or urgent.
For planned procedures, different strategies are proposed based on the indication for APAs. Cardiologists and anesthesiologists often share similar practices, balancing thrombotic and hemorrhagic risks, though cardiologists may focus more on thrombotic risks, while anesthesiologists prioritize hemorrhagic risks.
The therapeutic approach is inherently multidisciplinary, involving anesthesiologists, interventional cardiologists, cardiologists, and surgeons.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations Ethics approval: The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
References:
- Godier A., Fontana P., Motte S., Steib A., Bonhomme F., Schlumberger S., et al. Management of antiplatelet therapy in patients undergoing elective invasive procedures. Proposals from the French Working Group on perioperative haemostasis (GIHP) and the French Study Group on thrombosis and haemostasis (GFHT). In collaboration with the French Society for Anaesthesia and Intensive Care Medicine (SFAR). Anaesth Crit Care Pain Med. 2018;37:379-389. [PubMed.]
- Devereaux PJ., Mrkobrada M., Sessler DI., Leslie K., Alonso-Coello P., Kurz A., et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–503. [PubMed.]
- Oscarsson A., Gupta A., Fredrikson M., Jarhult J., Nystrom M., Pettersson E., et al. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. British Journal of anaesthesia. 2010;104:305–12. [PubMed.]
- Holcomb CN., Graham LA., Richman JS., Rhyne RR., Itani KMF., Maddox TM., et al. The incremen- tal risk of noncardiac surgery on adverse cardiac events following coronary stenting. Journal of the American College of Cardiology. 2014;64:2730–9. [PubMed.]
- Holcomb CN., Hollis RH., Graham LA., Richman JS., Valle JA., Itani KM., et al. Association of Coronary Stent Indication with Postoperative Outcomes Following Noncardiac Surgery. JAMA Surg. 2016;151:462–8. [PubMed.]
- Filipescu DC., Stefan MG., Valeanu L., Popescu WM. Perioperative management of antiplatelet therapy in noncardiac surgery. Curr Opin Anaesthesiol. 2020;33(3):454-462. [PubMed.]
- Rossini R., Musumeci G., Visconti LO., Bramucci E., Castiglioni B., et al. Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and noncardiac surgery: a consensus document from Italian cardiological., surgical and anaesthesiological societies. EuroIntervention. 2014;10(1):38-46. [PubMed.]
- Shah S., Urtecho M., Firwana M., Nayfeh T., Hasan B., Nanaa A., et al. Perioperative Management of Antiplatelet Therapy: A Systematic Review and Meta-analysis. Mayo Clin Proc Innov Qual Outcomes. 2022;6(6):564-573. [PubMed.]