>Corresponding Author : Madhuri S Kurdi
>Article Type : Short Communication Article
>Volume : 2 | Issue : 2
>Received Date : 20 April, 2022
>Accepted Date : 30 April, 2022
>Published Date : 03 May, 2022
>DOI : https://doi.org/10.54289/JAAD2200106
>Citation : Kurdi MS, Joshi V, Ladhad D, Bhat R, Athira GS, et al. (2022) Anaesthetic Management of a Case of Sinonasal Mucormycosis with Post Covid -19 Pulmonary Embolism for Endoscopic Debridement. J Anaesth Anesth Drug 2(2): doi https://doi.org/10.54289/JAAD2200106
>Copyright : © 2022 Kurdi MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Short Communication Article | Open Access | Full Text
Department of Anaesthesiology, Karnataka Institute of Medical Sciences (KIMS), Hubli, Karnataka, India
*Corresponding author: Madhuri S Kurdi, Department of Anaesthesiology, Karnataka Institute of Medical Sciences (KIMS), Hubli, Karnataka, India
Short Communication
Coronavirus disease (COVID)-19, especially the severe disease is associated with an increased risk and prevalence of venous thromboembolism [1].
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) binds to the angiotensin converting enzyme-2 (ACE-2) receptors on endothelial cells, especially within the kidneys, heart, lungs, etc. causing endothelial cell damage leading to thrombosis and thrombotic complications like deep vein thrombosis, pulmonary embolism, myocardial infarction, etc [2].We present here our experience of the anaesthetic management of a 40 year old male with post COVID-19 sinonasal mucormycosis and history of pulmonary embolism posted for bilateral functional endoscopic sinus surgery (FESS) and endoscopic debridement. The patient had developed COVID-19 with a computed tomography (CT) severity score on high resolution computed tomography (HRCT) of 16/25. One month post COVID-19 positive status, he was diagnosed with pulmonary embolism clinically and this was confirmed by a CT pulmonary angiogram (Figure1). He was put on non-invasive ventilation (NIV) in the intensive care unit (ICU) for seven days, weaned off and put on non-rebreather mask (NRBM) for four days and then put on simple face mask with 5L oxygen flow per minute. During this period, thrombolysis was done for pulmonary embolism, and anti-coagulation was continued with oral Factor Xa inhibitor apixaban 5mg twice daily for 14 days. On pre-anaesthetic evaluation, room air saturation and six minute walk test were 93% and 90% respectively.
The patient was posted for surgery after optimisation. Apixaban was stopped three days preoperatively. Chest auscultation revealed bilateral normal vesicular breath sounds. Other haemodynamic variables and blood parameters including coagulation profile were within normal limits. Chest radiogram showed bilateral haziness and 2D echocardiography revealed normal findings. After adequate preoxygenation and premedication with intravenous fentanyl and midazolam, patient was induced with propofol and succinylcholine and endotracheal intubation with 8.0 mm internal diameter cuffed tube was done. Intraoperatively, high peak airway pressures were observed (38 to 40 cmH2O). Hence, pressure control mode was used for lung protective ventilation. With a peak inspiratory pressure of 24 to 28 cmH2O, only 350 ml of tidal volume could be generated and end-tidal carbon dioxide (EtCO2) was 49 to 54 mmHg. So, the peak airway pressure was increased to 34 cm H2O to achieve a tidal volume of 400ml and a compliance of 17ml/cm of H2O, after which the EtCO2 came down to 32 to 37 mmHg (Figure2). Anaesthesia was maintained with sevoflurane (minimum alveolar concentration 0.5-1.5), oxygen: nitrous oxide (50:50). Haemodynamic stability and hydration were maintained throughout the surgery. Intra-operative blood loss was minimal. The extubation and post operative period were uneventful. Apixaban was restarted two days post operatively.
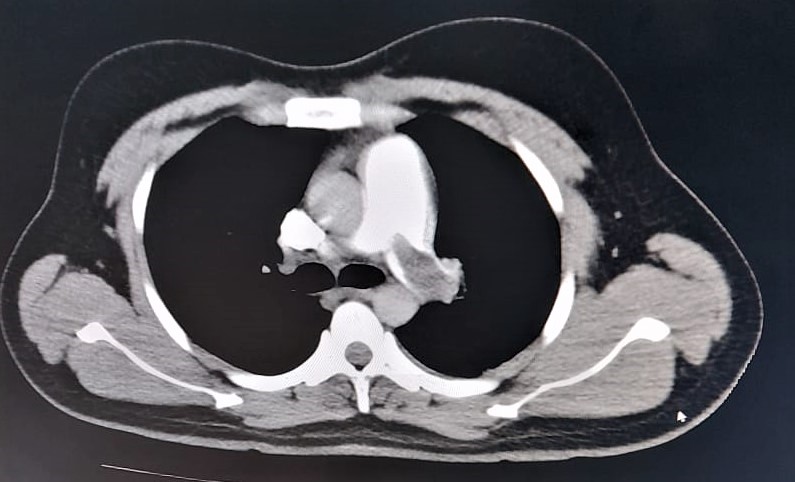
Figure 1: CT pulmonary angiogram showing a filling defect in the left main pulmonary artery – a sign of thrombosis
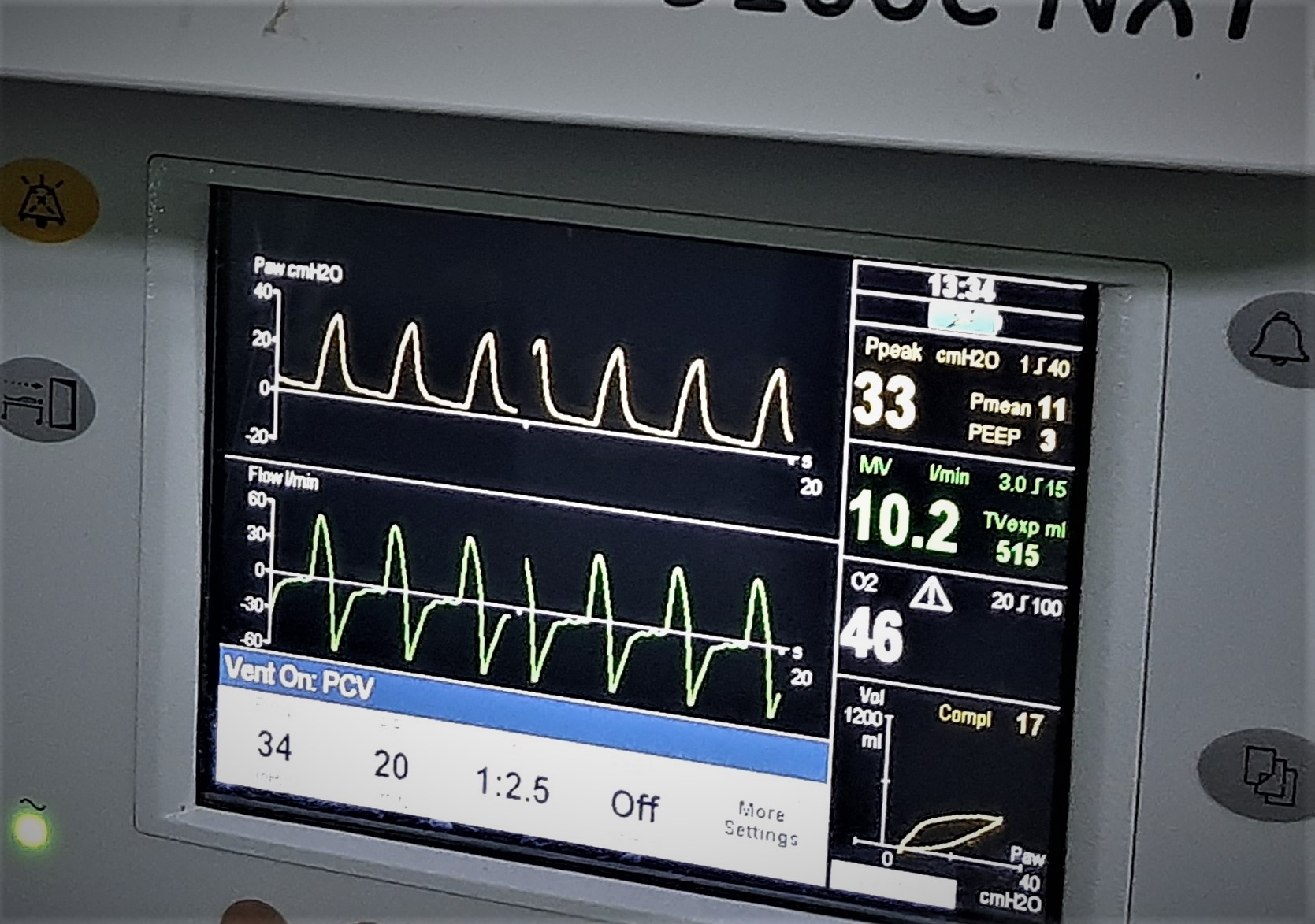
Figure 2: Ventilator waveform of pressure-controlled ventilation with pressure support of 34 cm H2O generating an expiratory tidal volume of 515ml, peak airway pressure of 33 cm H2O and low lung compliance.
Severe COVID-19 patients have all the three elements of Virchow’s triad: endothelial injury, stasis and hypercoagulable state contributing as risk factors for thromboembolism [3]. However, pulmonary embolism has also been noted in patients with COVID-19 without any other standard risk factors suggesting that COVID -19 itself is an independent risk factor for thromboembolism.
Rehabilitation and multidisciplinary optimisation including chest physiotherapy, incentive spirometry, use of bronchodilators, correction of hydration status, improvement of nutrition, control of blood sugars and anti-thrombotic therapy are recommended for the successful perioperative outcome of post-COVID-19 pulmonary embolism patients [4].
Intraoperative anaesthetic considerations include adequate preoxygenation (use of transnasal humidified rapid insufflation ventilatory exchange (THRIVE) for apnoeic oxygenation), avoidance of drugs causing myocardial depression, optimisation of preload and afterload and lung protective ventilation strategies. As these patients are at high risk of re-embolism and subsequent cardiovascular and neurological adverse effects, advanced monitoring like trans-oesophageal echocardiogram and cardiac doppler are suggested. Nevertheless, these monitors were not used in our case.
Though there are various guidelines for the optimal timing of surgery in post COVID -19 patients, currently there are no established guidelines regarding the perioperative management of a patient with history of post COVID-19 pulmonary embolism. This is especially with regard to the timing of surgery in relation to thrombolysis and preoperative optimisation of the lungs. Hence, extended research into these aspects is required.
References
- Vechi HT, Maia LR, Alves MD. (2020) Late acute pulmonary embolism after mild coronavirus disease 2019 (COVID-19): a case series. Revista do Instituto de Medicina Tropical de São Paulo. 4: 62-71. [Ref.]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, Stevens JS, et al. (2021) Post-acute COVID-19 syndrome. Nature medicine. 27: 601-615. [Ref.]
- Vadukul P, Sharma DS, Vincent P. (2020) Massive pulmonary embolism following recovery from COVID-19 infection: inflammation, thrombosis and the role of extended thromboprophylaxis. BMJ case reports CP. 13(9): e238168. [PubMed.]
- Malhotra N, Bajwa SJ, Joshi M, Mehdiratta L, Hemantkumar I, et al. (2021) Perioperative management of post-COVID-19 surgical patients: Indian society of anaesthesiologists (ISA national) advisory and position statement. Indian Journal of Anaesthesia. 65(7): 499-507. [Ref.]