>Corresponding Author : Sarah M Bobker
>Article Type : Research Article
>Volume : 2 | Issue : 1
>Received Date : 31 March, 2022
>Accepted Date : 10 April, 2022
>Published Date : 14 April, 2022
>DOI : https://doi.org/10.54289/JAAD2200105
>Citation : Bobker SM, Ehrlich A, Recchioni C, Levin M, Riggins N. (2022) Retrospective Chart Review: The Feasibility of a Self-Administered Nasal Spray Targeting the Sphenopalatine Ganglion (SPG) in Treatment of Chronic Migraine. J Anaesth Anesth Drug 2(1): doi https://doi.org/10.54289/JAAD2200105
>Copyright : © 2022 Bobker SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Research Article | Open Access | Full Text
UCSF Headache Center, San Francisco, USA
*Corresponding author: Sarah M Bobker, UCSF Headache Center, San Francisco, USA
Abstract
Objective: To describe the results of a self-administered anesthetic nasal spray developed to target the sphenopalatine ganglion (SPG) for the acute and preventive treatment of chronic migraine.
Background: The SPG is a known migraine treatment target that may be anesthetized via minimally invasive in-office procedures. Historically, various noninvasive selfadministered intranasal anesthetic formulations have been developed for the treatment of migraine, with some studies suggesting implication of the SPG mechanism, however, this has needed further development and data on efficacy has been mixed and inconclusive. During COVID-19 pandemic clinic closures, we noticed a substantial need for improved at-home therapies for our university headache center patients with chronic migraine.
Methods: We developed a compounded anesthetic nasal spray utilizing upward force and a supine delivery positioning with the aim of better targeting the SPG. Patients were instructed to use this treatment once per month as prevention and, additionally, up to four times per month as need acutely. Retrospective chart review was performed. Physician clinical judgment of patient reports was used to assign a dichotomous conclusion of the usefulness of such treatment for patients at an interval of 3 months (preventive effect) and acutely (acute effect). Adverse effects were also reviewed. Results differentiate treatment response in medication overuse headache (MOH) and peripartum subgroups as well.
Results: 52 out of 66 (79%) patients reported improvement in overall headache frequency or intensity at 3 month follow up (preventive effect). 40 out of 53 (75%) patients who using the treatment acutely reported improvement in headache intensity (acute effect). Of the 6 patients also with MOH, 5 (83%) reported preventive effect and 4 (67%) reported acute effect. Of the 9 patients who were also peripartum, all 9 (100%) reported preventive effect and 8 (89%) reported acute effect. No significant adverse effects were reported.
Conclusion: A self-administered nasal spray developed to target the SPG may be an effective and safe therapy for the acute and preventive treatment of chronic migraine. This delivery method suggests safe and potentially successful blockade of the SPG at home. Further developments in non-invasive intranasal techniques are warranted.
Keywords: Sphenopalatine Ganglion Block; Chronic Migraine; Prevention; Acute Treatment
Abbreviations: SPG: Sphenopalatine Ganglion, IRB: Institutional Review Board, MOH: Medication Overuse Headache, ONBs: Occipital Nerve Blocks, TPIs: Trigger Point Injections, TCC: Trigeminocervical Complex
Introduction
Migraine is a highly prevalent neurological disease associated with substantial disability and suffering worldwide [1]. It is characterized by paroxysmal, multiphase attacks of head pain accompanied by other neurological symptoms (namely hypersensitivities, to visual, auditory, and/or olfactory stimuli, nausea and vomiting) [2,3]. Chronic migraine is estimated to occur in 2% of the general population [3] and is distinctly defined in the International Classification of Headache Disorders-3 (ICHD-3) by headaches occurring on at least 15 days per month for more than 3 months, with at least 8 days a month on which headaches and associated symptoms meet diagnostic criteria for migraine [4].
Chronic migraine is particularly challenging to treat. Approach to treatment incorporates several domains, including addressing lifestyle and risk factor modifications, preventive treatments (both pharmacological and non-pharmacological modalities), and abortive treatments. Often minimally invasive procedures performed in-office may be useful adjunctive therapies, including occipital nerve blocks (ONBs), trigger point injections (TPIs), and SPG blocks.
The SPG is an aggregate of neurons lying deep in the bilateral pterygopalatine fossae of the skull and is a known target for the treatment of migraine [5]. It carries important parasympathetic outflow to the craniofacial structures, including possible activation of the trigeminocervical complex (TCC) that is believed to play a role in headache [6]. Local anesthetization (“blocking”) of the SPG is believed to modulate sensory processing and thereby reduce central sensitization of pain in migraine.
Practitioners may perform SPG blocks by direct percutaneous injection [7] through an area on the cheek or by transnasal-directed catheters or cotton swabs. Various noninvasive intranasal anesthetic formulations and delivery methods have historically been developed in attempt to treat migraine, namely acute attacks, however, the evidence for such has been mixed and inconclusive [8]. This may be, at least in part, due to the fact that the distance from the nasal mucosa to the SPG appears to be longer than was previously assumed [7] and, is therefore, harder to target noninvasively.
It was Maizels et al in 1996 who first reported via a randomized, double blind, controlled trial that intranasal lidocaine provided rapid relief of headache (at least 50% reduction of headache within 15 minutes after treatment) in approximately 55% of ambulatory patients with migraine [9]. However, this study did not clearly address the use and dosage of this medication and likely gave itself away by a higher placebo response rate since intranasal lidocaine is irritating and likely easily distinguished from the relatively comfortable saline that was used as control.
In 2018, Dagenais and Zed performed a systematic review to evaluate the efficacy and safety of intranasal lidocaine in the acute management of primary headaches and discovered some pitfalls: first, potential benefit over placebo in acute pain reduction (and in the need for rescue medication) was seen only in four studies; second, no study reported benefit in preventing headache recurrence nor repeat visits to the emergency department; and, third, lidocaine was associated with significantly higher rates of adverse events compared with placebo and may have resulted in lower rates of patient satisfaction [10].
To contrast, in 2019, Chi et al conducted a meta-analysis of randomized controlled trials investigating the efficacy of intranasal lidocaine compared with placebo or an active comparator and discovered intranasal lidocaine may be considered a useful option for patients with acute migraine with high success rate, low pain intensity, infrequent need for rescue medication, and tolerable adverse events noted [11].
For the purpose of this study, we focus attention on a noninvasive, selfadministered approach that we developed while clinics were closed during COVID-19 [11]. With a gap in procedural treatments available to our chronic migraine patients during this time [12], we were inspired by previous intranasal lidocaine investigations and a hope to closely target the SPG at home. Our hypothesis was that this delivery method was safe and potentially successful in self-administering anesthetic to the SPG region.
Methods:
At the start of the COVID-19 pandemic, our team worked closely with our university’s pharmacy and a local specialty pharmacy to develop a spray bottle device potentially able to deliver anesthetic more precisely toward the SPG region. This spray bottle utilized an upward force delivery (Figure 1). Solutions of 1% Lidocaine, 2% Lidocaine, or 0.5% Ropivacaine were made available (Figure 2). The medication was delivered to the patient’s home for convenience.
Patients were instructed to spray once in one nostril and then lie on their back with their head tilted toward the ipsilateral side of administration for 5 minutes; then to repeat this procedure on the contralateral side. Bilateral use constituted a single treatment. The patient’s physician and pharmacy provided specific written and verbal instructions for use in order to control appropriate technique as much as possible.
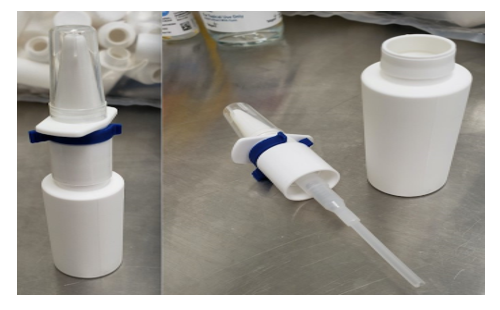
Figure 1
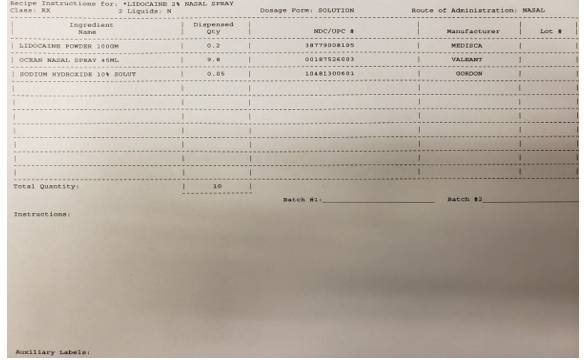
Figure 2: Example recipe instructions for Lidocaine 2% Nasal Spray formulation.
Treatment was recommended one time monthly as a preventive treatment and, additionally, up to four more times monthly as needed as acute treatment. This “five uses per month” designation of the 0.1cc spray dose was calculated based on the standard SPG block protocol used at our headache center, wherein a 0.5cc dose may be administered via in-office SPG per nostril per month. Therefore, 0.5cc divided by 0.1cc available nasal spray arrived us at "five uses per month”.
Persons with chronic migraine as defined by the ICHD-3 were identified as potential candidates for this treatment and provided a prescription at the discretion of their clinician. A retrospective chart review of all available patient charts receiving this treatment from May 1, 2020, through March 1, 2021, with described time frame of at least a 3-month treatment window, was conducted. The only exclusion criterion was not having a 3-month interval follow up available to review in the chart. Two different practitioners at our headache center performed independent review of all charts and physician clinical judgment of patient reports were used to assign dichotomous conclusions on response preventively and response acutely. All dichotomous conclusions between both providers were found concordant. Reported side effects were also noted.
Due to the small sample size and the observational nature of this data, formal quantitative statistical analysis was not performed. Descriptive statistical analysis was utilized instead. Retrospective chart review of outpatient charts at our center is approved under institutional review board (IRB) #14-14862.
Results:
66 patients were identified for inclusion in this study. Table 1 reports patient demographics. Most were female and the mean age was 48 years old. On average, patients had previously tried 6 different acute medications and 10 different preventive medications, which had either not provided enough benefit to be continued or caused intolerable side effects.
Physician clinical judgment of patient reports was used to assign a dichotomous conclusion on the usefulness as a preventive treatment, at an interval of 3 months, in 79% (52/66) and as an acute treatment in 75% (40/53) of patients. No significant adverse effects were reported, although two patients did temporarily experience migraine exacerbation after use and discontinued further treatment. As expected, some patients experienced transient and mild, well-tolerated numbness in and around the area of anesthetic application.
Table 1: Demographic variables
| Table 1- Patient Demographics |
|---|
|
Total patients included: 66
Used the treatment preventively: 66 (100%) Used the treatment acutely: 53 (80%) Female: 51 (77%) Male: 15 (23%) Mean age: 48 years, SD (15.09), Range (20 to 84) Concomitant diagnosis of MOH: 6 (9%) Peripartum (pregnant, planning pregnancy, or breastfeeding): 9 (18%) |
Results also differentiate MOH and peripartum subgroups. Of those also with MOH, 83% (5/6) reported preventive effect and 67% (4/6) also reported acute effect. To note further, the 4 patients who found this treatment helpful acutely also responded preventively. Of those patients who were also peripartum, 100% (9/9) reported preventive effect and 89% (8/9) reported acute effect.
Discussion:
We identify this as a pilot proof-of-concept study that seems to further suggest the possibility of a noninvasive, self-administered anesthetic treatment successfully targeting SPG. To our knowledge, this is the first report to indicate that SPG blockade at home may provide effective treatment preventively in migraine.
Although we acknowledge that there are several limitations to this work, there are some outcomes to highlight that we feel further add to this topic in the literature. It is worth noting first that our population represents a very treatment-resistant one, yet that many did clinically respond to this treatment. Further, clinical improvement was reported in a high percentage of those also with MOH (although we acknowledge the sample size is quite small). Success too was seen too in peripartum patients, notably when fewer migraine treatments are available and safe for use.
Major limitations of this study include lack of a rigorous study design and small sample size with resultant descriptive, rather than quantitative, statistical analysis.
We attribute this largely to the circumstances of the COVID-19 pandemic. We believe this work has relevance when thought of as a pilot, feasibility study. Future studies should be done to look at a larger sample size and incorporate control group. Further, although these results are favourable and the delivery and administration method perhaps unique, we also acknowledge that we are unable to definitively prove that the SPG has been reached (“blocked”) with this treatment.
The suggestion of noninvasive SPG blockade has been made historically in many other works, with these results further contributing to that conversation and calling for further treatment development. Future studies may too one day directly compare the efficacy and safety of such self-administered techniques with in-office procedures.
Conclusion:
A self-administered nasal spray developed to target the SPG may be an effective and safe therapy for the acute and preventive treatment of chronic migraine. This delivery method suggests safe and potentially successful blockade of the SPG at home. Further developments in noninvasive intranasal techniques are warranted.
Acknowledgements: Thank you to Henna Sawhney, our UCSF Headache Centre’s
Research Coordinator at the time of this work! Thank you to the involved pharmacists:
Bryan Hunt PharmD, Michelle Lee PharmD
References
- Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, et al. (2021) Migraine: epidemiology and systems of care. Lancet. 397(10283): 1485-1495. [PubMed.]
- Dodick DW. (2018) Migraine. Lancet. 391(10127): 1315-1330. [Ref.]
- Schwedt TJ. (2014) Chronic migraine. BMJ. 348: g1416. [PubMed.]
- Headache Classification Committee of the International Headache Society (IHS). (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 33(9): 629-808. [PubMed.]
- Mojica J, Mo B, Ng A. (2017) Sphenopalatine Ganglion Block in the Management of Chronic Headaches. Curr Pain Headache Rep. 21(6): 27. [PubMed.]
- Robbins MS, Robertson CE, Kaplan E, Ailani J, Kuruvilla D, et al. (2016) The Sphenopalatine Ganglion: Anatomy, Pathophysiology, and Therapeutic Targeting in Headache. 56(2): 240-258. [PubMed.]
- Crespi J, Bratbak D, Dodick D, Matharu M, Jamtoy KA, et al. (2018) Measurement and implications of the distance between the sphenopalatine ganglion and nasal mucosa: a neuroimaging study. J Headache Pain. 19(1): 14. [PubMed.]
- Maizels M. (1999) Intranasal lidocaine to prevent headache following migraine aura. Headache. 39(6): 439-442. [PubMed.]
- Maizels M, Scott B, Cohen W, Chen W, et al. (1996) Intranasal lidocaine for treatment of migraine: a randomized, double-blind, controlled trial. JAMA. 276(4): 319-321. [PubMed.]
- Dagenais R, Zed PJ. (2018) Intranasal Lidocaine for Acute Management of Primary Headaches: A Systematic Review. Pharmacotherapy. 38(10): 1038-1050. [PubMed.]
- Chi PW, Hsieh KY, Chen KY, Hsu CW, Bai CH, et al. (2019) Intranasal lidocaine for acute migraine: A meta-analysis of randomized controlled trials. PLoS One. 14(10): e0224285. [PubMed.]
- Szperka CL, Ailani J, Barmherzig R, Klein BC, Singh RBH, et al. (2020) Migraine Care in the Era of COVID-19: Clinical Pearls and Plea to Insurers. Headache. 60(5): 833-842 [PubMed.]